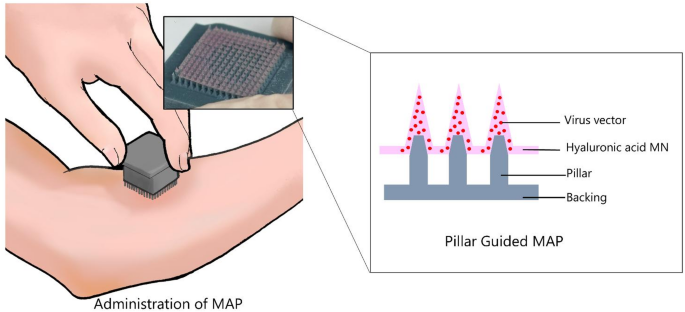
Preparation of vaccine solution
The recombinant vaccinia virus strain r-DIs-S, carrying the SARS-CoV-2 spike gene, was produced at the Tokyo Metropolitan Institute of Medical Science. (Tokyo, JAPAN). To prepare the vaccine solution, 30 wt% hyaluronic acid with a molecular weight of 14 kDa (Contipro a.s., Dolní Dobrouč, CZECH REPUBLIC) and 10% trehalose (T9531-10G, Sigma-Aldrich, St. Louis, USA) were added to a suspension of r-DIs-S virus (1.3 × 109 PFU/mL in DMEM (10569-010, Thermo Fisher Scientific, Waltham, US)), and the mixture was gently dissolved at 4 °C by pipetting.
To optimize the HA concentration, four formulations containing 5%, 10%, 15%, and 20% HA (w/v) were prepared. Trehalose was maintained at a constant concentration of 5% (w/v) across all formulations. HA powder was dissolved in Dulbecco’s Modified Eagle Medium (DMEM) at 25 °C for 6 h, and the virus solution was added to a final titer of 1 × 10⁷ PFU/mL. Each 500 µL sample was transferred to sterile 1.5 mL microcentrifuge tubes and stored at 4 °C for 6 h. Viral titers were determined using a plaque assay.
To assess the effects of temperature on viral stability during MAP fabrication, 15% HA solutions containing r-DIs-S (initial titer: 1.0 × 105 PFU/mL) were prepared and stored at 4–25 °C for 6 h to simulate dispensing and drying conditions during MAP fabrication. After this incubation period, the samples were subjected to plaque assays.
Fabrication of PG-MAP
A 14 × 14 microneedle master mold was fabricated from tungsten carbide using electrical discharge machining (EDM). The mold geometry was confirmed by optical microscopy (VH-5500; Keyence, Osaka, JAPAN) and image analysis (Zeiss ZEN, Carl Zeiss AG, Oberkochen, GERMANY). A polydimethylsiloxane (PDMS) negative mold was cast from the master mold.
The MAP backing layer was designed using 3D CAD software (Fusion 360, version 2604.1.48, https://www.autodesk.com/products/fusion-360, Autodesk, Inc., San Francisco, USA) and fabricated using a stereolithography (SLA) 3D printer (Saturn 2, Elegoo, Shenzhen, CHINA) with a water-washable resin (8 K, Elegoo, Shenzhen, CHINA). After printing, the backings were rinsed in DI water and ethanol, UV-cured (Mercury Plus V2.0, Elegoo, Shenzhen, CHINA), and oven-dried at 40 °C for 1 h to remove residual photoinitiators. The dimensions were determined such that the top cover would fit inside the bottom mold, with the back-layer thickness of the MAPs ranging from 500 to 550 μm.
Next, a PDMS negative mold fabricated from the master mold was treated with air plasma (YHS-R, Sakigake, Tokyo, JAPAN) to enhance the adhesion between the viscous hyaluronic acid r-DIs-S solution and the PDMS mold surface. This plasma treatment was used to improve the wettability of the substrate with the vaccine solution. 50 µl of mixed formulation including 15 wt % HA and vaccinia virus (1.0 × 10⁸ PFU mL⁻¹) solutions were then dispensed onto the plasma-treated PDMS mold. After aligning and positioning the prefabricated backing layer with an r-DIs-S solution-filled PDMS mold, the assembled patches were placed in a sealed container with a portable dehumidifier and dried in a refrigerator at 4 °C and 10% RH for 8 h to ensure complete microneedle drying. After complete drying, the backing layer was carefully removed from the PDMS mold.
All fabrication was performed in a biosafety cabinet (MHE-131AJ, Sanyo, Osaka, JAPAN) at 25 °C. MAPs were dried in a sealed container with a portable dehumidifier (MD-3, Toyo Living, Tokyo, JAPAN) at 4 °C for 8 h to ensure complete drying of the microneedles.
Drying kinetics: One-step vs. Pillar guided methods
To evaluate the drying kinetics, MAPs were fabricated using both conventional one-step molding and the pillar-guided method. A 15% HA solution was used. For one-step molding, 1.0 mL of solution was added to each PDMS mold, whereas for the pillar-guided method, only 50 µL was used. The mold–backing assemblies were weighed on an analytical balance (SECURA125-1SJP, Sartorius AG, Göttingen, GERMANY) to obtain the initial mass (m₀) at 1-h intervals. Drying time was defined as the duration required to achieve three consecutive weights varying by n = 2) were dried either in a refrigerated chamber at 4 °C (10% RH) or at room temperature (25 °C, 10% RH).
Theoretical Estimation of Tip-Loading efficiency (TLE)
Tip-loading efficiency (TLE) was calculated from the geometric parameters of the microneedle array, which means the calculations from the microneedle volume (Vneedle) and total dispensed volume (Vtotal). The internal volume of a single microneedle (Vneedle) was obtained from master mold parameters. Vtips is the total volume of 14 × 14 microneedle cavities, therefore
$$\:{V}_{tips}\text{}=196\times\:{V}_{needle}$$
TLE was then expressed as
$$\:TLE\:\left(t{\%}\right)=\frac{{V}_{tips}}{\text{}{V}_{total}}\text{}\text{}\times\:100,$$
where Vtotal is the solution volume dispensed into the mold (1 mL for the one-step method and 50 µL for the pillar-guided method). This estimate assumes full cavity filling and no bridging.
Viral titer after MAP fabrication
To evaluate viral retention, the microneedles were fully dissolved in 1 mL of DMEM and incubated for 5 min at room temperature. The resulting solution was immediately used for plaque assays.
Mechanical strength testing
The mechanical failure forces of the microneedles were measured using a force gauge (MX2-500 N, Imada, Aichi, JAPAN). A compressive force was applied until individual needle failure was detected, ensuring penetration into the skin layer without breaking.
Kinetics release
PG-MAP were subjected to a release kinetics study. PG-MAP containing 1% (w/w) Rhodamine B were dissolved in 20 mL of PBS(-) pH 7.4 at 37 °C under stirring at 100 rpm to ensure uniform distribution and maintain equilibrium conditions. At predetermined time points ranging from 0.5 to 30 min, 200 µL were collected for analysis. The concentration of the released Rhodamine B was measured by UV spectrophotometry (UV-2600, Shimadzu Corporation, Tokyo, Japan) within the 500–580 nm spectrum range. After each measurement, 200 µL of PBS(-) pH 7.4 solution was replaced within the sealed container to maintain a constant volume. Finally, the release profile was plotted with the y-axis representing the absorbance of rhodamine B as a function of time to visualize the dissolution behavior of the PG-MAP.
Plaque assay
Plaque assays for vaccine stability testing were performed as follows. Chicken embryonic cells (CRL-12203 UMNSAH/DF-1 cells, ATCC, Manassas, USA) were seeded in a 6-well plate at a density of 8 × 105 cells/well and incubated at 37 °C and 5% CO2 in a humidified atmosphere for 24 h.
For each sample, the viral solution was serially diluted in a maintenance medium containing 2% fetal bovine serum (FBS) (A525671, Thermo Fisher Scientific, Waltham, US), and 100 µL of each viral solution was dispensed into the designated wells and incubated at 37 °C and 5% CO2 in a humidified atmosphere for 1 h. Overlay medium (0.5%/MC/DMEM + GlutaMAX/5%FBS) was prepared from DMEM (10569-010, Thermo Fisher Scientific, Waltham, US) supplemented with Methylcellulose (22224-55, NACALAI TESQUE, Kyoko, JAPAN), 5% FBS (A525671, Thermo Fisher Scientific, Waltham, US), penicillin-streptomycin (PS) (15140-122, Thermo Fisher Scientific, Waltham, US), and HEPES(1 M) (15630-080, Thermo Fisher Scientific, Waltham, US) in distilled water). Overlay medium (2 mL/well) was added to each well of the titration plate and incubated at 37 °C in a 5% CO2 incubator for 3 days.
The overlay medium was then carefully aspirated, and the cells in each well were gently washed with PBS. Cells were fixed by adding 2 mL of 10% neutral buffered formalin to each well and incubated in a biosafety cabinet for at least 1 h. Following fixation, formalin was carefully removed, and each well was washed twice with 1 mL of tap water. Subsequently, 2 mL of tap water was added to each well, the plate lids were opened, and the plates were exposed to UV light within a biosafety cabinet for 1 h to ensure viral inactivation. After UV treatment, crystal violet solution (2 mL) was added to each well and incubated at room temperature for 15 min. Virus dilution factors representing 5–100 plaques per well were identified, and the virus titer was calculated by counting the plaques.
Ethics statement
All animal experiments complied with the Science Council of JAPAN’s and ARRIVE guidelines for the proper conduct of animal research. Protocols were approved by the Animal Care and Use Committee of the Tokyo Metropolitan Institute of Medical Science (Approval No. 24–071).
Animal vaccination
Negative BALB/c mice (8 weeks old; JAPAN SLC Inc.) were maintained under a 12-h light/dark cycle with ad libitum access to food and water. The mice were anesthetized with intraperitoneal ketamine/xylazine (150 µL) and shaved dorsally.
A pre-vaccination blood sample (50 µL) was collected from the retro-orbital venous plexus, and the baseline serum was separated and stored at −20 °C.
Fifteen mice were randomly assigned to three groups (n = 5 per group). In the first control group, mice received 5 µL of sterile phosphate-buffered saline (PBS) via skin-scarification. In the second group, two MAPs containing (6.14–6.78 × 106 PFU) recombinant vaccinia virus DIs strains carrying the SARS-CoV-2 S gene (r-DIs-S) were applied to the shaved dorsal skin (one patch on the right upper quadrant and one on the left lower quadrant) for 5 min. In the third group, mice received 1 × 107 PFU of the DIs virus in 5 µL via skin scarification.
After PG-MAP application, the PG-MAP was carefully removed. The PG-MAP before and after insertion was observed under a microscope to count the number of microneedles retained in the patch versus those inserted into the skin. The puncture rate was calculated, and the delivered dose was estimated based on the initial number of microneedles and the virus titer. Viral titers in the dissolved microneedles (after patch retrieval) were evaluated using a plaque assay, as described previously. The second vaccination was administered at week 4 using the same procedure. Additional blood samples were collected from the submandibular vein at Weeks 0,2,4 and 6 for immunological analysis.
Evaluation of immunogenicity
To assess the immune induction after vaccination, we performed the following experiments.
Enzyme-linked immunosorbent assay (ELISA)
IgG responses against inactivated r-DIs and SARS-CoV-2 spike proteins were quantified using standard ELISA protocols. Plates were coated with NP40-inactivated DIs or recombinant S protein (Acro, SPN-C52H8), followed by blocking, incubation with diluted serum, secondary HRP-conjugated antibody, substrate development (OPD), and absorbance measurement at 492 nm. Details are as follows. DIs virus was grown in primary chicken fibroblasts and purified using OptiPrep™ (Iodixanol) density gradient medium and ultracentrifugation. Purified DIs were suspended in PBS and inactivated with 0.2% NP40. The inactivated DIs solution was coated onto 96-well round-bottom plates and incubated overnight at 4 °C. For IgG detection against the SARS-CoV-2 spike protein, 96-well plates were coated by incubating them overnight at 4 °C with 1 µg/mL of recombinant C-terminus His-tagged spike protein of the early pandemic strain of SARS-CoV-2 (Acro, #SPN-C52H8) dissolved in 50 mM carbonate buffer (pH 9.6). The plates were blocked with 1% BSA in PBS(-), containing 0.5% Tween20 and 2.5 mM ethylene diaminetetra-acetic acid, followed by incubation with a 100- or 1000-fold dilution of sera collected from vaccinated mice. After extensive washing, the plates were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG polyclonal antibody (62–6520, Thermo Fisher Scientific, Waltham, MA, US). Next, 100 µL of a mixture of o-phenylenediamine dihydrochloride and hydrogen peroxide (H2O2) in citrate–phosphate buffer was added to each well. The reactions were quenched by adding 1 M sulfuric acid, and the absorbance was measured at 492 nm wavelength.
In vitro neutralization assay for vaccinia virus
To assess neutralizing antibody activity, mouse sera were heat-inactivated at 56 °C for 30 min. Serial dilutions of heat-inactivated sera were mixed with equal volumes of 3000 PFU/mL DIs and incubated at 37 °C overnight. DF-1 cells were then infected with 30 µL of the virus/serum mixture in 24-well plates. At 96 h after infection, the neutralization titer was expressed as the reciprocal of the maximum dilution of serum at which the plaque numbers were reduced by 50% compared to those in wells infected with the virus alone (without serum).
Challenge experiment with SARS-CoV-2
Ten weeks after the 2nd vaccination, the mice were inoculated intranasally with the SARS-CoV-2 TY8-612 strain (GISAID ID: EPI_ISL_1123289), lineage B. 1. 351, provided by Dr. Masayuki Saijo, Mutsuyo Takayama-Ito, and Masaaki Satoh (Department of Virology 1, National Institute of Infectious Diseases)38. Each mouse received 1 × 105 PFU in a 50 µL volume. Body weight was monitored daily, and mice that lost more than 30% of their initial body weight were humanely euthanized and recorded as deceased.
The animal was euthanized by cervical dislocation immediately after blood collection via cardiac puncture under isoflurane anesthesia. Subsequently, the lungs were harvested via thoracotomy. The collected blood was centrifuged (15,000 rpm, 5 min, RT), and the serum was recovered. Both the serum and lungs were stored at − 80 °C until use.
Determination of infectious SARS-CoV-2 titer
Infectious SARS-CoV-2 titers (viral loads) were determined using a standard plaque assay. Briefly, the left lung lobes were harvested, homogenized, and centrifuged. The supernatants were stored at − 80 °C. Infectious titers were determined by plaque assay on Vero E6/TMPRSS2 cells using 0.6% agarose overlays and crystal violet staining. The details are as follows. The left lung lobe from each necropsied mouse was weighed and homogenized in nine volumes of Hanks-balanced saline solution (Thermo Fisher Scientific, Waltham, MA, USA) using a Multi-Beads Shocker (YASUI KIKAI, Osaka, JAPAN). Homogenates were centrifuged at 3000 × g for 10 min at 4 °C. The supernatant was collected and stored at − 80 °C until further use. For the plaque assay, serial tenfold dilutions of the supernatant (100 µL per well) were inoculated onto confluent monolayers of Vero E6/TMPRSS2 cells in a 6-well plate and incubated at 37 °C for 1 h. Unbound viruses were removed by washing the cells with DMEM. The cells were then overlaid with DMEM containing 10% FBS and 0.6% agarose (Sigma-Aldrich, St. Louis, MO, USA). After 72 h of incubation at 37 °C, the cells were fixed in 10% neutral buffered formalin and stained with 1% crystal violet. The titer of SARS-CoV-2 was defined as plaque-forming units per gram of lung tissue (PFU/g lung), with a detection limit of 100 PFU/g lung.
SARS-CoV-2 RNA quantification
Total RNA was extracted from 70 µL of the supernatant from lung homogenates prepared for the Plaque Assay (see plaque assay) using RNeasy Mini kits (Qiagen, Hilden, GERMANY) according to the manufacturer’s instructions. RT-qPCR targeting the SARS-CoV-2 N gene was performed using a CDC primer/probe set (N2-F, N2-R, and N2-P). Fifty nanograms of total RNA was used to quantify the SARS-CoV-2 N protein gene39. The primers and probe used were as follows: forward primer 5’- TTACAAACATTGGCCGCAAA-3’ (2019-SARS-CoV-2_N2-F), reverse primer 5’- GCGCGACATTCCGAAGAA-3’ (2019-SARS-CoV-2_N2-R), and probe 5’-FAM- ACAATTTGCCCCCAGCGCTTCAG-BHQ1-3’ (2019-SARS-CoV-2_N2-P). Viral RNA quantification was performed using a 1-step reverse transcription-quantitative polymerase chain reaction (RT-qPCR), as previously described38. Viral loads were calculated as Log10 copies per microgram of total RNA.
Lung histopathology
The upper lobe of the right lung from each necropsied mouse was fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned to a thickness of 4 μm, stained with hematoxylin and eosin (H&E), and subjected to routine histological examination. Images of H&E-stained sections were obtained using an all-in-one fluorescence microscope (BZ-X710, KEYENCE, Osaka, JAPAN) equipped with a Plan Apo 20x Lambda objective (NA0.75, Nikon, Tokyo, JAPAN).
Statistical analysis
Data plotted on a linear scale are expressed as the mean ± standard deviation (SD), while those plotted on a logarithmic scale are expressed as the geometric mean ± geometric SD. Inferential statistical analyses were performed using one-way ANOVA, followed by Tukey’s test, as appropriate. Statistical significance was set at p https://www.graphpad.com, GraphPad Software, Inc., Boston, USA).