Louis, D. N. et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 23, 1231–1251 (2021).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2016–2020. Neuro Oncol. 25, iv1–iv99 (2023).
White, K. et al. New hints towards a precision medicine strategy for IDH wild-type glioblastoma. Ann. Oncol. 31, 1679–1692 (2020).
Yabo, Y. A., Niclou, S. P. & Golebiewska, A. Cancer cell heterogeneity and plasticity: a paradigm shift in glioblastoma. Neuro Oncol. 24, 669–682 (2022).
Galassi, C., Chan, T. A., Vitale, I. & Galluzzi, L. The hallmarks of cancer immune evasion. Cancer Cell 42, 1825–1863 (2024).
Galluzzi, L., Smith, K. N., Liston, A. & Garg, A. D. The diversity of CD8+ T cell dysfunction in cancer and viral infection. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-025-01161-6 (2025).
Wu, B., Zhang, B., Li, B., Wu, H. & Jiang, M. Cold and hot tumors: from molecular mechanisms to targeted therapy. Signal Transduct. Target. Ther. 9, 274 (2024).
Neftel, C. et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell 178, 835–849 (2019).
Wang, L. et al. A single-cell atlas of glioblastoma evolution under therapy reveals cell-intrinsic and cell-extrinsic therapeutic targets. Nat. Cancer 3, 1534–1552 (2022).
Patel, A. P. et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401 (2014).
Dirkse, A. et al. Stem cell-associated heterogeneity in glioblastoma results from intrinsic tumor plasticity shaped by the microenvironment. Nat. Commun. 10, 1787 (2019).
Wang, Q. et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell 32, 42–56 (2017).
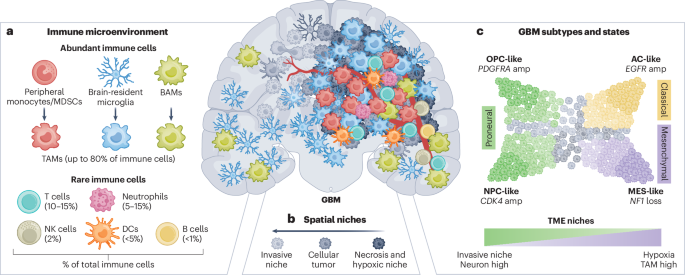
Greenwald, A. C. et al. Integrative spatial analysis reveals a multi-layered organization of glioblastoma. Cell 187, 2485–2501 (2024).
Nomura, M. et al. The multilayered transcriptional architecture of glioblastoma ecosystems. Nat. Genet. 57, 1155–1167 (2025).
Faust Akl, C. et al. Glioblastoma-instructed astrocytes suppress tumour-specific T cell immunity. Nature 643, 219–229 (2025).
Wälchli, T. et al. Single-cell atlas of the human brain vasculature across development, adulthood and disease. Nature 632, 603–613 (2024).
Khan, F. et al. Macrophages and microglia in glioblastoma: heterogeneity, plasticity, and therapy. J. Clin. Invest. https://doi.org/10.1172/JCI163446 (2023).
Sharma, P., Aaroe, A., Liang, J. & Puduvalli, V. K. Tumor microenvironment in glioblastoma: current and emerging concepts. Neurooncol. Adv. 5, vdad009 (2023).
Bikfalvi, A. et al. Challenges in glioblastoma research: focus on the tumor microenvironment. Trends Cancer 9, 9–27 (2023); erratum 9, 692 (2023).
Habashy, K. J., Mansour, R., Moussalem, C., Sawaya, R. & Massaad, M. J. Challenges in glioblastoma immunotherapy: mechanisms of resistance and therapeutic approaches to overcome them. Br. J. Cancer 127, 976–987 (2022).
Arrieta, V. A. et al. Immune checkpoint blockade in glioblastoma: from tumor heterogeneity to personalized treatment. J. Clin. Invest. https://doi.org/10.1172/JCI163447 (2023).
Bunse, L., Bunse, T., Kilian, M., Quintana, F. J. & Platten, M. The immunology of brain tumors. Sci. Immunol. 10, eads0449 (2025).
Mahdi, J., Trivedi, V. & Monje, M. The promise of immunotherapy for central nervous system tumours. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-025-01227-5 (2025).
Hambardzumyan, D. & Bergers, G. Glioblastoma: defining tumor niches. Trends Cancer 1, 252–265 (2015).
Dermitzakis, I. et al. CNS border-associated macrophages: ontogeny and potential implication in disease. Curr. Issues Mol. Biol. 45, 4285–4300 (2023).
Mrdjen, D. et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380–395 (2018).
Friebel, E. et al. Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell 181, 1626–1642 (2020).
Pombo Antunes, A. R. et al. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat. Neurosci. 24, 595–610 (2021).
Ochocka, N. et al. Single-cell RNA sequencing reveals functional heterogeneity of glioma-associated brain macrophages. Nat. Commun. 12, 1151 (2021).
Yabo, Y. A. et al. Glioblastoma-instructed microglia transition to heterogeneous phenotypic states with phagocytic and dendritic cell-like features in patient tumors and patient-derived orthotopic xenografts. Genome Med. 16, 51 (2024).
Klemm, F. et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell 181, 1643–1660 (2020).
Miller, T. E. et al. Programs, origins and immunomodulatory functions of myeloid cells in glioma. Nature https://doi.org/10.1038/s41586-025-08633-8 (2025).
González-Tablas Pimenta, M. et al. Tumor cell and immune cell profiles in primary human glioblastoma: impact on patient outcome. Brain Pathol. 31, 365–380 (2021).
Bayik, D. et al. Myeloid-derived suppressor cell subsets drive glioblastoma growth in a sex-specific manner. Cancer Discov. 10, 1210–1225 (2020).
Jackson, C. et al. Distinct myeloid-derived suppressor cell populations in human glioblastoma. Science 387, eabm5214 (2025).
Zhao, J. et al. Disease-specific suppressive granulocytes participate in glioma progression. Cell Rep. 43, 115014 (2024).
Maas, R. R. et al. The local microenvironment drives activation of neutrophils in human brain tumors. Cell 186, 4546–4566 (2023).
Yang, Y. et al. Large-scale bulk and single-cell RNA sequencing combined with machine learning reveals glioblastoma-associated neutrophil heterogeneity and establishes a VEGFA+ neutrophil prognostic model. Biol. Direct 20, 45 (2025).
Chen, Z. et al. Monocyte depletion enhances neutrophil influx and proneural to mesenchymal transition in glioblastoma. Nat. Commun. 14, 1839 (2023).
Chanoch-Myers, R., Wider, A., Suva, M. L. & Tirosh, I. Elucidating the diversity of malignant mesenchymal states in glioblastoma by integrative analysis. Genome Med. 14, 106 (2022).
Rashidi, A. et al. Myeloid cell-derived creatine in the hypoxic niche promotes glioblastoma growth. Cell Metab. 36, 62–77 (2024).
Mitsdoerffer, M. et al. The glioblastoma multiforme tumor site promotes the commitment of tumor-infiltrating lymphocytes to the TH17 lineage in humans. Proc. Natl Acad. Sci. USA 119, e2206208119 (2022).
Andaloussi, A. E. & Lesniak, M. S. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 8, 234–243 (2006).
Mohme, M. et al. Immunophenotyping of newly diagnosed and recurrent glioblastoma defines distinct immune exhaustion profiles in peripheral and tumor-infiltrating lymphocytes. Clin. Cancer Res. 24, 4187–4200 (2018).
van Hooren, L. et al. Agonistic CD40 therapy induces tertiary lymphoid structures but impairs responses to checkpoint blockade in glioma. Nat. Commun. 12, 4127 (2021).
Cakmak, P. et al. Spatial immune profiling defines a subset of human gliomas with functional tertiary lymphoid structures. Immunity 58, 2025–2863.e2848 (2025).
Wang, F. et al. Comparison of tumor immune environment between newly diagnosed and recurrent glioblastoma including matched patients. J. Neurooncol. 159, 163–175 (2022).
Breznik, B. et al. Infiltrating natural killer cells bind, lyse and increase chemotherapy efficacy in glioblastoma stem-like tumorospheres. Commun. Biol. 5, 436 (2022).
Kmiecik, J. et al. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J. Neuroimmunol. 264, 71–83 (2013).
Hou, D. et al. Antigen-presenting B cells promote TCF-1+ PD1− stem-like CD8+ T-cell proliferation in glioblastoma. Front. Immunol. 14, 1295218 (2024).
Gao, J. et al. Infiltrating plasma cells maintain glioblastoma stem cells through IgG–tumor binding. Cancer Cell https://doi.org/10.1016/j.ccell.2024.12.006 (2024).
Woroniecka, K. et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin. Cancer Res. 24, 4175–4186 (2018).
Chongsathidkiet, P. et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat. Med. 24, 1459–1468 (2018).
Magri, S. et al. Sustained accumulation of blood-derived macrophages in the immune microenvironment of patients with recurrent glioblastoma after therapy. Cancers (Basel) 13, 6178 (2021).
Pires-Afonso, Y. et al. Elucidating tumour-associated microglia/macrophage diversity along glioblastoma progression and under ACOD1 deficiency. Mol. Oncol. 16, 3167–3191 (2022).
Kirschenbaum, D. et al. Time-resolved single-cell transcriptomics defines immune trajectories in glioblastoma. Cell 187, 149–165 (2024).
Sattiraju, A. et al. Hypoxic niches attract and sequester tumor-associated macrophages and cytotoxic T cells and reprogram them for immunosuppression. Immunity 56, 1825–1843 (2023).
Lohr, J. et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin. Cancer Res. 17, 4296–4308 (2011).
Dobersalske, C. et al. Cranioencephalic functional lymphoid units in glioblastoma. Nat. Med. 30, 2947–2956 (2024).
Koh, B. I. et al. Adult skull bone marrow is an expanding and resilient haematopoietic reservoir. Nature 636, 172–181 (2024).
Du, L. et al. Both IDO1 and TDO contribute to the malignancy of gliomas via the Kyn–AhR–AQP4 signaling pathway. Signal Transduct. Target. Ther. 5, 10 (2020).
Takenaka, M. C. et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 22, 729–740 (2019).
Didenko, V. V., Ngo, H. N., Minchew, C. & Baskin, D. S. Apoptosis of T lymphocytes invading glioblastomas multiforme: a possible tumor defense mechanism. J. Neurosurg. 96, 580–584 (2002).
Badie, B., Schartner, J., Prabakaran, S., Paul, J. & Vorpahl, J. Expression of Fas ligand by microglia: possible role in glioma immune evasion. J. Neuroimmunol. 120, 19–24 (2001).
Butt, N. S. et al. Major histocompatibility class-I (MHC-I) downregulation in glioblastoma is a poor prognostic factor but not a predictive indicator for treatment failure. Pathol. Res. Pract. 250, 154816 (2023).
Yang, W., Li, Y., Gao, R., Xiu, Z. & Sun, T. MHC class I dysfunction of glioma stem cells escapes from CTL-mediated immune response via activation of Wnt/β-catenin signaling pathway. Oncogene 39, 1098–1111 (2020).
Zhang, M. et al. Anti-CD47 treatment stimulates phagocytosis of glioblastoma by M1 and M2 polarized macrophages and promotes M1 polarized macrophages in vivo. PLoS ONE 11, e0153550 (2016).
Wu, M. et al. Phagocytosis of glioma cells enhances the immunosuppressive phenotype of bone marrow-derived macrophages. Cancer Res. 83, 771–785 (2023).
Schmassmann, P. et al. Targeting the Siglec–sialic acid axis promotes antitumor immune responses in preclinical models of glioblastoma. Sci. Transl. Med. 15, eadf5302 (2023).
von Roemeling, C. A. et al. Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity. Nat. Commun. 11, 1508 (2020).
Mei, Y. et al. Siglec-9 acts as an immune-checkpoint molecule on macrophages in glioblastoma, restricting T-cell priming and immunotherapy response. Nat. Cancer 4, 1273–1291 (2023).
Gordon, S. R. et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495–499 (2017).
Jadus, M. R. et al. Macrophages kill T9 glioma tumor cells bearing the membrane isoform of macrophage colony stimulating factor through a phagocytosis-dependent pathway. J. Immunol. 160, 361–368 (1998).
Afzal, A. et al. Phagocytosis checkpoints in glioblastoma: CD47 and beyond. Curr. Issues Mol. Biol. 46, 7795–7811 (2024).
Kopatz, J. et al. Siglec-h on activated microglia for recognition and engulfment of glioma cells. Glia 61, 1122–1133 (2013).
Kim, H.-J. et al. Blood monocyte-derived CD169+ macrophages contribute to antitumor immunity against glioblastoma. Nat. Commun. 13, 6211 (2022).
Saavedra-López, E. et al. Phagocytic glioblastoma-associated microglia and macrophages populate invading pseudopalisades. Brain Commun. 2, fcz043 (2020).
Friedrich, M. et al. Dysfunctional dendritic cells limit antigen-specific T cell response in glioma. Neuro Oncol. 25, 263–276 (2023).
Carenza, C. et al. Perioperative corticosteroid treatment impairs tumor-infiltrating dendritic cells in patients with newly diagnosed adult-type diffuse gliomas. Front. Immunol. 13, 1074762 (2022).
Chang, A. L. et al. CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 76, 5671–5682 (2016).
Guan, X. et al. CTLA4-mediated immunosuppression in glioblastoma is associated with the infiltration of macrophages in the tumor microenvironment. J. Inflamm. Res. 14, 7315–7329 (2021).
Abdelfattah, N. et al. Single-cell analysis of human glioma and immune cells identifies S100A4 as an immunotherapy target. Nat. Commun. 13, 767 (2022).
Lerner, E. C. et al. CD8+ T cells maintain killing of MHC-I-negative tumor cells through the NKG2D–NKG2DL axis. Nat. Cancer 4, 1258–1272 (2023).
Hu, X. et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 30, 229–243 (2020).
Song, E. et al. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 577, 689–694 (2020).
Blobner, J. et al. Comparative evaluation of T-cell receptors in experimental glioma-draining lymph nodes. Neurooncol. Adv. 3, vdab147 (2021).
Ravi, V. M. et al. T-cell dysfunction in the glioblastoma microenvironment is mediated by myeloid cells releasing interleukin-10. Nat. Commun. 13, 925 (2022).
Miller, B. C. et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019).
Polania, J. W. et al. Antigen presentation by tumor-associated macrophages drives T cells from a progenitor exhaustion state to terminal exhaustion. Immunity https://doi.org/10.1016/j.immuni.2024.11.026 (2024).
Kilian, M. et al. MHC class II-restricted antigen presentation is required to prevent dysfunction of cytotoxic T cells by blood-borne myeloids in brain tumors. Cancer Cell 41, 235–251 (2023).
Vijayanathan, Y. & Ho, I. A. W. The impact of metabolic rewiring in glioblastoma: the immune landscape and therapeutic strategies. Int. J. Mol. Sci. 26, 669 (2025).
De Leo, A. et al. Glucose-driven histone lactylation promotes the immunosuppressive activity of monocyte-derived macrophages in glioblastoma. Immunity 57, 1105–1123 (2024).
Wang, S. et al. Oncolytic viruses engineered to enforce cholesterol efflux restore tumor-associated macrophage phagocytosis and anti-tumor immunity in glioblastoma. Nat. Commun. 14, 4367 (2023).
Governa, V. et al. Protumoral lipid droplet-loaded macrophages are enriched in human glioblastoma and can be therapeutically targeted. Sci. Transl. Med. 16, eadk1168 (2024).
Kloosterman, D. J. et al. Macrophage-mediated myelin recycling fuels brain cancer malignancy. Cell 187, 5336–5356 (2024).
Huff, W. X. et al. Aging- and tumor-mediated increase in CD8+CD28− T cells might impose a strong barrier to success of immunotherapy in glioblastoma. Immunohorizons 5, 395–409 (2021).
Scharping, N. E. et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat. Immunol. 22, 205–215 (2021).
Lin, A. J. et al. Impact of concurrent versus adjuvant chemotherapy on the severity and duration of lymphopenia in glioma patients treated with radiation therapy. J. Neurooncol. 136, 403–411 (2018).
Karachi, A. et al. Modulation of temozolomide dose differentially affects T-cell response to immune checkpoint inhibition. Neuro Oncol. 21, 730–741 (2019).
Varn, F. S. et al. Glioma progression is shaped by genetic evolution and microenvironment interactions. Cell 185, 2184–2199 (2022).
Spitzer, A. et al. Deciphering the longitudinal trajectories of glioblastoma ecosystems by integrative single-cell genomics. Nat. Genet. 57, 1168–1178 (2025).
Loussouarn, D. et al. Spatial distribution of immune cells in primary and recurrent glioblastoma: a small case study. Cancers (Basel) 15, 3256 (2023).
Shekarian, T. et al. Multidimensional analysis of matched primary and recurrent glioblastoma identifies contributors to tumor recurrence influencing time to relapse. J. Neuropathol. Exp. Neurol. 84, 45–58 (2024).
Onubogu, U. et al. Spatial analysis of recurrent glioblastoma reveals perivascular niche organization. JCI Insight https://doi.org/10.1172/jci.insight.179853 (2024).
van Hooren, L. et al. CD103+ regulatory T cells underlie resistance to radio-immunotherapy and impair CD8+ T cell activation in glioblastoma. Nat. Cancer 4, 665–681 (2023).
Tamura, R. et al. Alterations of the tumor microenvironment in glioblastoma following radiation and temozolomide with or without bevacizumab. Ann. Transl. Med. 8, 297 (2020).
Wang, W. et al. Glioblastoma pseudoprogression and true progression reveal spatially variable transcriptional differences. Acta Neuropathol. Commun. 11, 192 (2023).
Knudsen, A. M. et al. Characterisation of the tumour microenvironment in primary and recurrent glioblastomas. Neuropathol. Appl. Neurobiol. 50, e13012 (2024).
Touat, M. et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature 580, 517–523 (2020).
Gromeier, M. et al. Very low mutation burden is a feature of inflamed recurrent glioblastomas responsive to cancer immunotherapy. Nat. Commun. 12, 352 (2021).
Akkari, L. et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci. Transl. Med. 12, eaaw7843 (2020).
Watson, S. S. et al. Fibrotic response to anti-CSF-1R therapy potentiates glioblastoma recurrence. Cancer Cell 42, 1507–1527 (2024).
Watson, S. S. et al. Microenvironmental reorganization in brain tumors following radiotherapy and recurrence revealed by hyperplexed immunofluorescence imaging. Nat. Commun. 15, 3226 (2024).
Ha, W. et al. Ibudilast sensitizes glioblastoma to temozolomide by targeting macrophage migration inhibitory factor (MIF). Sci. Rep. 9, 2905 (2019).
Sørensen, M. D. et al. Microglia induce an interferon-stimulated gene expression profile in glioblastoma and increase glioblastoma resistance to temozolomide. Neuropathol. Appl. Neurobiol. 50, e13016 (2024).
Yeo, A. T. et al. Single-cell RNA sequencing reveals evolution of immune landscape during glioblastoma progression. Nat. Immunol. 23, 971–984 (2022).
Tamura, R. et al. Persistent restoration to the immunosupportive tumor microenvironment in glioblastoma by bevacizumab. Cancer Sci. 110, 499–508 (2019).
Wei, Q. et al. TNFα secreted by glioma associated macrophages promotes endothelial activation and resistance against anti-angiogenic therapy. Acta Neuropathol. Commun. 9, 67 (2021).
Aslan, K. et al. Heterogeneity of response to immune checkpoint blockade in hypermutated experimental gliomas. Nat. Commun. 11, 931 (2020).
Lee-Chang, C. et al. Myeloid-derived suppressive cells promote B cell-mediated immunosuppression via transfer of PD-L1 in glioblastoma. Cancer Immunol. Res. 7, 1928–1943 (2019).
Chryplewicz, A. et al. Cancer cell autophagy, reprogrammed macrophages, and remodeled vasculature in glioblastoma triggers tumor immunity. Cancer Cell 40, 1111–1127 (2022).
Klein, E., Hau, A.-C., Oudin, A., Golebiewska, A. & Niclou, S. P. Glioblastoma organoids: pre-clinical applications and challenges in the context of immunotherapy. Front. Oncol. 10, 604121 (2020).
Steindl, A. & Valiente, M. Potential of ex vivo organotypic slice cultures in neuro-oncology. Neuro Oncol. 27, 338–351 (2025).
Wang, G. & Wang, W. Advanced cell therapies for glioblastoma. Front. Immunol. https://doi.org/10.3389/fimmu.2022.904133 (2022).
Choi, B. D., Maus, M. V., June, C. H. & Sampson, J. H. Immunotherapy for glioblastoma: adoptive T-cell strategies. Clin. Cancer Res. 25, 2042–2048 (2019).
Bagley, S. J., Desai, A. S., Linette, G. P., June, C. H. & O’Rourke, D. M. CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges. Neuro Oncol. 20, 1429–1438 (2018).
Khan, S. M. et al. Impact of CD4 T cells on intratumoral CD8 T-cell exhaustion and responsiveness to PD-1 blockade therapy in mouse brain tumors. J. Immunother. Cancer https://doi.org/10.1136/jitc-2022-005293 (2022).
Reardon, D. A. et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 6, 1003–1010 (2020).
Lim, M. et al. Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro Oncol. 24, 1935–1949 (2022).
Duerinck, J. et al. Intracerebral administration of CTLA-4 and PD-1 immune checkpoint blocking monoclonal antibodies in patients with recurrent glioblastoma: a phase I clinical trial. J. Immunother. Cancer 9, e002296 (2021).
Omuro, A. et al. Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: an international randomized phase III trial. Neuro Oncol. 25, 123–134 (2023).
Lee, A. H. et al. Neoadjuvant PD-1 blockade induces T cell and cDC1 activation but fails to overcome the immunosuppressive tumor associated macrophages in recurrent glioblastoma. Nat. Commun. 12, 6938 (2021).
Schalper, K. A. et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat. Med. 25, 470–476 (2019).
Cloughesy, T. F. et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 25, 477–486 (2019).
Zhao, J. et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat. Med. 25, 462–469 (2019).
Begley, S. L., O’Rourke, D. M. & Binder, Z. A. CAR T cell therapy for glioblastoma: a review of the first decade of clinical trials. Mol. Ther. https://doi.org/10.1016/j.ymthe.2025.03.004 (2025).
Goutnik, M. et al. Advancements in chimeric antigen receptor-expressing T-cell therapy for glioblastoma multiforme: literature review and future directions. Neurooncol. Adv. 6, vdae025 (2024).
Hatae, R. et al. Enhancing CAR-T cell metabolism to overcome hypoxic conditions in the brain tumor microenvironment. JCI Insight https://doi.org/10.1172/jci.insight.177141 (2024).
Brown, C. E. et al. Locoregional delivery of IL-13Rα2-targeting CAR-T cells in recurrent high-grade glioma: a phase 1 trial. Nat. Med. 30, 1001–1012 (2024).
Choi, B. D. et al. Intraventricular CARv3-TEAM-E T cells in recurrent glioblastoma. N. Engl. J. Med. 390, 1290–1298 (2024).
Bagley, S. J. et al. Intrathecal bivalent CAR T cells targeting EGFR and IL13Rα2 in recurrent glioblastoma: phase 1 trial interim results. Nat. Med. 30, 1320–1329 (2024).
Chih, Y.-C. et al. Vaccine-induced T cell receptor T cell therapy targeting a glioblastoma stemness antigen. Nat. Commun. 16, 1262 (2025).
Singh, K. et al. IL-7-mediated expansion of autologous lymphocytes increases CD8+ VLA-4 expression and accumulation in glioblastoma models. J. Clin. Invest. https://doi.org/10.1172/jci181471 (2025).
Klichinsky, M. et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 38, 947–953 (2020).
Martins, T. A. et al. Enhancing anti-EGFRvIII CAR T cell therapy against glioblastoma with a paracrine SIRPγ-derived CD47 blocker. Nat. Commun. 15, 9718 (2024).
Hutter, G. et al. Microglia are effector cells of CD47–SIRPα antiphagocytic axis disruption against glioblastoma. Proc. Natl Acad. Sci. USA 116, 997–1006 (2019).
Gholamin, S. et al. Irradiation or temozolomide chemotherapy enhances anti-CD47 treatment of glioblastoma. Innate Immun. 26, 130–137 (2020).
Amoozgar, Z. et al. Targeting Treg cells with GITR activation alleviates resistance to immunotherapy in murine glioblastomas. Nat. Commun. 12, 2582 (2021).
Galvez-Cancino, F. et al. Regulatory T cell depletion promotes myeloid cell activation and glioblastoma response to anti-PD1 and tumor-targeting antibodies. Immunity 58, 1236–1253 (2025).
Pyonteck, S. M. et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 19, 1264–1272 (2013).
Butowski, N. et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol. 18, 557–564 (2015).
Quail, D. F. et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science 352, aad3018 (2016).
Sun, R. et al. TREM2 inhibition triggers antitumor cell activity of myeloid cells in glioblastoma. Sci. Adv. 9, eade3559 (2023).
Peshoff, M. M. et al. Triggering receptor expressed on myeloid cells 2 (TREM2) regulates phagocytosis in glioblastoma. Neuro Oncol. 26, 826–839 (2024).
Zhong, J. et al. Distinct roles of TREM2 in central nervous system cancers and peripheral cancers. Cancer Cell 42, 968–984 (2024).
Lorimer, I. A. J. Potential roles for efferocytosis in glioblastoma immune evasion. Neurooncol. Adv. 6, vdae012 (2024).
Parker, S. et al. Immunotoxin–αCD40 therapy activates innate and adaptive immunity and generates a durable antitumor response in glioblastoma models. Sci. Transl. Med. 15, eabn5649 (2023).
Chen, D. et al. CTLA-4 blockade induces a microglia–Th1 cell partnership that stimulates microglia phagocytosis and anti-tumor function in glioblastoma. Immunity 56, 2086–2104 (2023).
Delamarre, L., Pack, M., Chang, H., Mellman, I. & Trombetta, E. S. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 307, 1630–1634 (2005).
Zhao, B., Kilian, M., Bunse, T., Platten, M. & Bunse, L. Tumor-reactive T helper cells in the context of vaccination against glioma. Cancer Cell 41, 1829–1834 (2023).
Sampson, J. H. et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 28, 4722–4729 (2010).
Liau, L. M. et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: a phase 3 prospective externally controlled cohort trial. JAMA Oncol. 9, 112–121 (2023).
Hilf, N. et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 565, 240–245 (2019).
Keskin, D. B. et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 565, 234–239 (2019).
Latzer, P. et al. A real-world observation of patients with glioblastoma treated with a personalized peptide vaccine. Nat. Commun. 15, 6870 (2024).
Tabatabai, G. et al. Treatment of glioblastoma patients with personalized vaccines outside clinical trials: lessons ignored? Neuro Oncol. https://doi.org/10.1093/neuonc/noae225 (2024).
Kim, T.-G. et al. Immunological factors relating to the antitumor effect of temozolomide chemoimmunotherapy in a murine glioma model. Clin. Vaccine Immunol. 17, 143–153 (2010).
Long, G. V. et al. Neoadjuvant triplet immune checkpoint blockade in newly diagnosed glioblastoma. Nat. Med. https://doi.org/10.1038/s41591-025-03512-1 (2025).
Ling, A. L. et al. Serial multiomics uncovers anti-glioblastoma responses not evident by routine clinical analyses. Sci. Transl. Med. 17, eadv2881 (2025).
Chevaleyre, C. et al. Efficient PD-L1 imaging of murine glioblastoma with FUS-aided immunoPET by leveraging FcRn–antibody interaction. Theranostics 13, 5584–5596 (2023).
Dar, D. et al. Imaging PD-L1 in the brain—journey from the lab to the clinic. Neuro Oncol. 27, 567–582 (2025).
Bettegowda, C. et al. Preanalytical variables and analytes in liquid biopsy approach for brain tumors: a comprehensive review and recommendations from the RANO Group and the Brain Liquid Biopsy Consortium. Neuro Oncol. https://doi.org/10.1093/neuonc/noaf140 (2025).
De Vleeschouwer, S. et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin. Cancer Res. 14, 3098–3104 (2008).