An early clinical trial is suggesting that directly injecting a virus-like, immune-activating compound into prostate tumors before surgery is safe and may help the innate and adaptive immune system recognize and attack cancer cells. The findings, published in Med: a Cell Press journal, demonstrate that neoadjuvant, sequential, guided injection of an intratumoral and intramuscular immunotherapy, polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose (poly-ICLC), is feasible and well-tolerated in men with intermediate-risk to high-risk localized prostate cancer.
Poly-ICLC is a synthetic double-stranded RNA molecule that acts as a pathogen-associated molecular pattern (PAMP) or “danger signal” to stimulate immune response. In an open-label phase 1 trial (Clinicaltrials.gov identifier: NCT03262103), 12 patients with clinically localized intermediate-risk to high-risk prostate cancer scheduled for radical prostatectomy (RP) received either 1 or 2 injections of poly-ICLC (Hiltonol®) into the prostate tumor to induce a local immune response followed by sequential intramuscular injections (eg, deltoid muscle) to boost the systemic response at weeks 3-6, respectively, for a progressively increasing number of cycles. All patients tolerated poly-ICLC without dose-limiting toxicity or treatment withdrawal. The median follow-up was 4.5 years. At 1 year after RP, 70% of the evaluable patients had a PSA of 0 (measured as PSA less than 0.1 ng/mL).
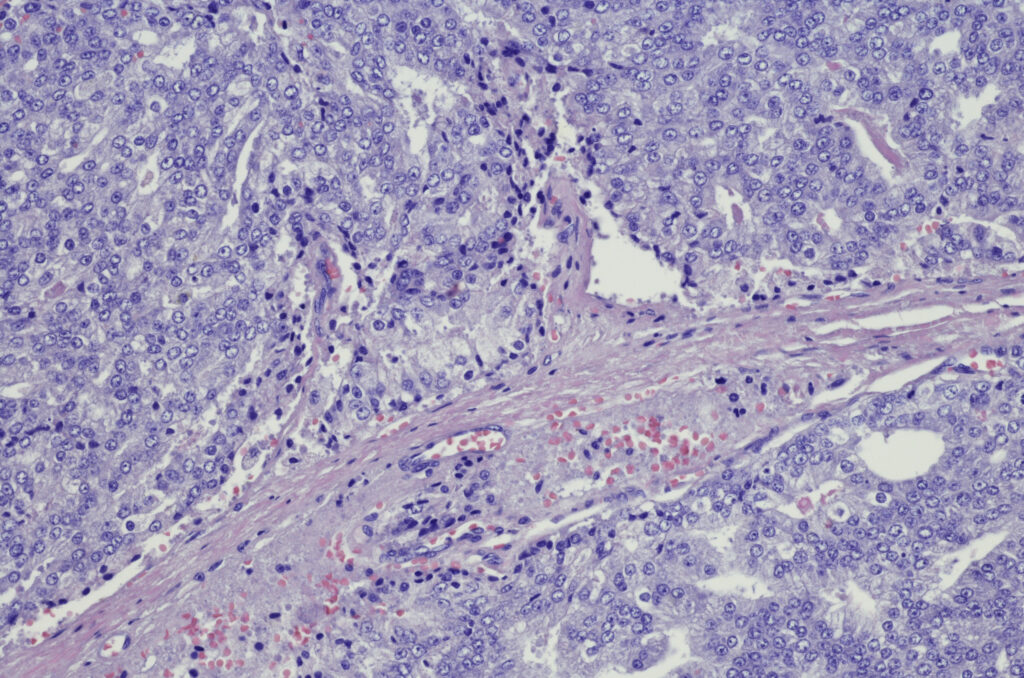
Gleason score at final pathology was downgraded in 66.7% of all patients and in 70% of the high-risk subgroup. Of 5 patients with clinical T3a or higher disease, 2 were downstaged to pT2 disease at RP. Tissue transcriptomic analysis revealed a decreased metastasis signature after treatment, with upregulation of immune cell-related and pro-apoptotic genes and downregulation of survival genes. Intratumoral and intramuscular poly-ICLC also enhanced immune activation signatures in the blood. Treatment increased infiltration of CD4+, CD8+, and PD-1+ T cells; CD56+ NK cells; CD20+ B cells; tertiary lymphoid structure-like aggregates associated with immunotherapy response; dendritic cells; as well as interferons, cytokines, chemokines, and costimulatory factors — all of which are associated with robust antitumor responses.
Study investigator Sujit S. Nair, PhD, who is an assistant professor and director of genitourinary immunotherapy research in the department of urology at the Icahn School of Medicine in New York City, said the autovaccine differs from an off-the-shelf vaccine because it uses the patient’s own tumor as the source of antigens. “We didn’t have to identify a specific target first. The tumor-directed immunization trains the immune system to recognize the whole tumor,” said Dr Nair, the lead and corresponding author of the paper. Poly-ICLC injection locally activates antigen-presenting dendritic cells. The dendritic cells then encounter tumor antigens from the dying tumor cells and present them, ultimately activating tumor antigen-specific T cells.
The treatment induced local and systemic immune activation and remodeled the tumor microenvironment. It also appeared to jump-start immune activity by drawing immune cells into the tumor, creating small pockets of immune cells where none had been before, and favorably shifting gene expression patterns.
Physician-scientist Ash Tewari, MD, of the department of urology at the Icahn School of Medicine pioneered the study. He reported that the treatment led to favorable biological signals and immune activation in the tissue and blood with encouraging pathology trends. “That suggests this kind of in situ immune priming could, in the future, help urologists better ‘condition’ the prostate before surgery and potentially support better oncologic outcomes,” said Dr Tewari, a co-corresponding study author. “The next step is a larger, controlled phase 2 study to confirm activity and define benefit. If those studies are positive, then broader adoption could be considered. So, for now, this should be viewed as an emerging, trial-based approach, and not for routine practice today.”
“In larger studies, we will need to keep monitoring for immune-related and local injection-related events, but the current safety profile is reassuring,” said Dr Nair. Poly-ICLC at a combined dose of 2.0 mg was well-tolerated. Treatment-emergent adverse events (TEAE) occurred in 7 of the 12 patients, primarily grade 1 injection-site soreness, fatigue, and flu-like symptoms plus 1 grade 3 fever. Treatment did not delay surgery or increase surgical complications.
By delivering poly-ICLC straight into the tumor under MRI-ultrasound fusion guidance, the researchers report they were able to engage local immunity before surgery. The same approach could be used to inject other immune agents or combinations, opening new paths for targeted treatment in the prostate.
If larger, longer-term studies confirm results, this strategy could help reprogram “cold” tumors into “immune-active” ones.
Co-corresponding author Nina Bhardwaj, MD, PhD, who is the director of the vaccine and cell therapy laboratory and co-director of the cancer immunology program at the Icahn School of Medicine, said this work shows that even tumors once thought to be invisible to the immune system can potentially be made responsive. “We plan to evaluate this strategy in a larger, controlled setting and in combination with other prostate cancer relevant therapies, to validate the immune and transcriptomic signals we observed. A phase 2 trial is the logical next step,” Dr Bhardwaj said. “If confirmed, this gives urologists a new approach to modulate the prostate tumor microenvironment and potentially improve the value of neoadjuvant strategies.” The team is now investigating the therapeutic benefit of poly-ICLC immunotherapy for patients with prostate cancer on active surveillance in a larger phase 2 randomized controlled trial (Clinicaltrials.gov identifier: NCT06343077).
Soroush Rais-Bahrami, MD, MBA, who is a professor and chair of the department of urology at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said this study is intriguing because it integrates concepts of immuno-oncologic sensitization with a tumor-targeted injection of an autovaccine. It is innovatively marrying multiple technological advances to prime prostate cancers for a precision medicine approach to treatment. “This phase 1, first-in-human clinical trial has capitalized on the longstanding efforts of vaccine development for cancer treatment with the guidance of software fusion to use MRI tumor localization for tumor-directed instillation of the autovaccine,” said Dr Rais-Bahrami. “I am hopeful that this and other efforts in this arena of discovery can help treat men with higher grade prostate cancer, known to be biologically more aggressive with higher rates of recurrence and metastasis, in whom body-wide immunologic priming may be key to improving outcomes.”
Alexander Kutikov, MD, who is chair of the Fox Chase Cancer Center department of urology in Philadelphia, Pennsylvania, said this approach has potential, but expectations should be guarded. “It’s a good space to watch, especially for combinations with checkpoint inhibitors or other therapies. However, immunotherapy work in the prostate cancer space thus far has largely been extremely disappointing,” said Dr Kutikov.
Urologist Jason Hafron, MD, a LUGPA board member from Troy, Michigan, said this year over 300,000 new cases of prostate cancer are projected to be diagnosed in the US, with about 15% classified as high-risk. “Approximately 45% to 65% of patients with high-risk disease experience recurrence within 5 years after RP. Treatment intensification remains a significant unmet need for these high-risk patients. Historically, prostate cancer has been regarded as a non-immunoactive tumor with limited responsiveness to immunotherapies. However, the authors show that they can modify the tumor microenvironment to promote an immune response, which is very exciting,” said Dr Hafron.
Disclosure: Some study authors declared affiliations with biotech, pharmaceutical, and/or device companies. Please see the original reference for a full list of authors’ disclosures.