A study from the University of California, published in Nature Communications, suggests that the toxoplasmosis parasite, present in about a third of the population, maintains active and organized cysts in the brain and muscles. Within these cysts, subtypes are divided: some sustain the chronic phase, while others prepare for reactivation precisely when immunity drops.
What seemed like “silence” within the body may, in practice, be a mode of operation. The parasite that causes toxoplasmosis can persist throughout life, and the new interpretation is unsettling: The parasite doesn’t just “stay there,” it organizes itself to continue existing..
This persistence occurs even when the initial phase passes without symptoms. In many cases, the immune system contains the beginning of the infection, but does not eliminate the parasite completely. It lodges itself in microscopic cysts, mainly in the brain and muscles, where conventional therapies are unable to reach effectively.
How the parasite enters, settles in, and becomes a long-term problem.
Contamination usually occurs through known routes: consumption of undercooked meat or contact with soil and feces of cats carrying the parasite. What varies from person to person is what happens next and, mainly, how much the immune system manages to “push” the parasite into a more controlled phase.
— ARTICLE CONTINUES BELOW —
Over time, the parasite forms cysts: structures surrounded by a protective layer and capable of harboring hundreds of bradyzoites, the form associated with the chronic phase. These cysts can reach 80 micrometers in size and frequently appear in neurons, as well as skeletal and cardiac muscles.
That’s when the story stops being about a “past infection” and becomes about permanent surveillance.
Why cysts are no longer seen as “inert”
For decades, the prevailing idea was simple: cysts were passive refuges, with dormant parasites, almost “frozen” in time. This reasoning shaped therapeutic strategies; if the parasite was inactive inside, it would be enough to control the acute phase and that would be it.
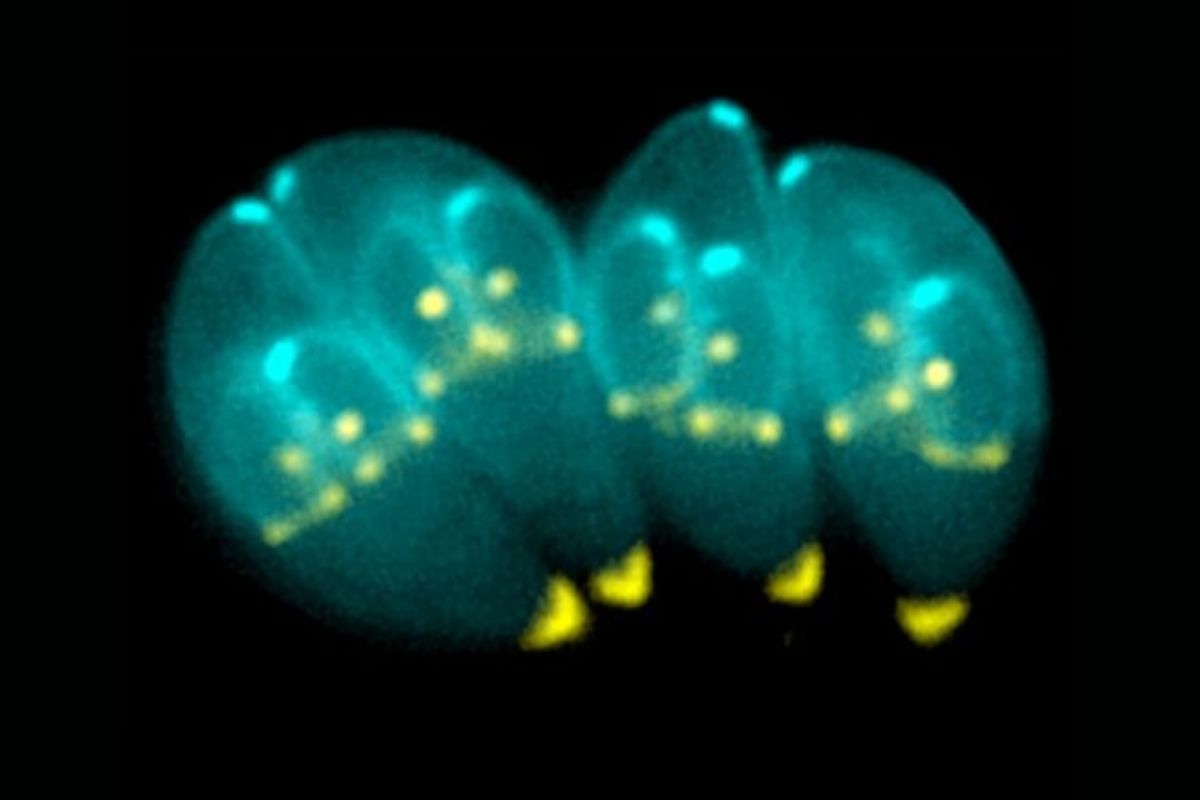
The new research points to a more complex scenario. By analyzing parasites extracted directly from cysts in living tissues, the team found an internal organization that does not match the notion of inertia.
The cyst begins to look less like a hiding place and more like a discreet “command center”., capable of responding to the host’s internal environment.
What single-cell RNA sequencing revealed inside the cyst
The turning point was methodological: using single-cell RNA sequencing, scientists identified multiple subtypes of bradyzoites within the cysts. All belong to the chronic phase, but with different biological functions, and this alone overturns the view that “a cyst is always the same.”
More than diversity, a pattern emerged: these subtypes were not mixed together by chance. There is a clear functional division.
Some appear adapted for long-term maintenance within the host; others are more geared towards transmission between hosts; and there are those that are ready to reactivate if immunological conditions change. In other words, the parasite distributes roles and the cyst becomes a dynamic structure.
When immunity drops, the change in form can reignite the infection.
This organization helps explain why toxoplasmosis can worsen under certain circumstances.
If an imbalance in the immune system occurs, the bradyzoites “prepared” for reactivation can transform into tachyzoites, the form linked to the rapid multiplication of the parasite.
When this happens, the infection can spread again throughout the body. The most feared effect is the possibility of toxoplasmic encephalitis and vision-threatening eye damage. The risk doesn’t arise from nothing: it can be “lying” inside the cyst, waiting for a weakness in the immune system.
What does this discovery change in the conversation about treatment?
Currently, available medications target tachyzoites. They can be effective in controlling the acute phase, but they do not impact the cysts. This creates a “therapeutic gap”: the chronic parasite remains protected, and medicine is, in practice, dependent on keeping the active phase under control when it appears.
The discovery of functional diversity within the cyst exposes an old problem: by treating the cyst as homogeneous and inactive, previous attempts to develop drugs may have ignored precisely what makes the infection persist.
The authors’ proposed interpretation is straightforward: understanding which subtypes are linked to reactivation can pave the way for more targeted therapies and potentially interrupt chronic infection with greater precision.
The traditional model of the parasite’s life cycle was often described as a simple alternation between an active and a latent phase.
However, if within the cyst there is an “ecosystem” of subtypes with different functions, the latency becomes less like an off mode and more like an intelligent standby mode.
The study’s lead researcher, Emma Wilson, summarizes this shift by advocating for a reassessment of the classic model: The cyst should be understood as the central point of control for the parasite..
This reorganizes research priorities and changes the practical question: it’s not just “how to kill the active parasite?”, but “how to disarm the parasite that remains ready inside the cyst?”.
The story of the parasite that “sleeps” in the brain takes on a different tone when science shows that it may, in fact, be preparing itself.
For a problem that affects about a third of the world’s population, treating the cyst as something static might be comforting, but perhaps unrealistic. The strongest message is that the chronic phase It’s not a lack of activity, it’s another type of strategy.
And you, when you read that a parasite can remain “organized” inside the body for years, does that change your perception of risk or does it seem like just a distant technical detail? If you could ask a doctor one thing about toxoplasmosis and immunity, what would it be and why?