February 4, 2026
3 min read
Lung cancer hijacks the brain to trick the immune system
Lung cancer tumor cells in mice communicate with the brain, sending signals to deactivate the body’s immune response, a study finds
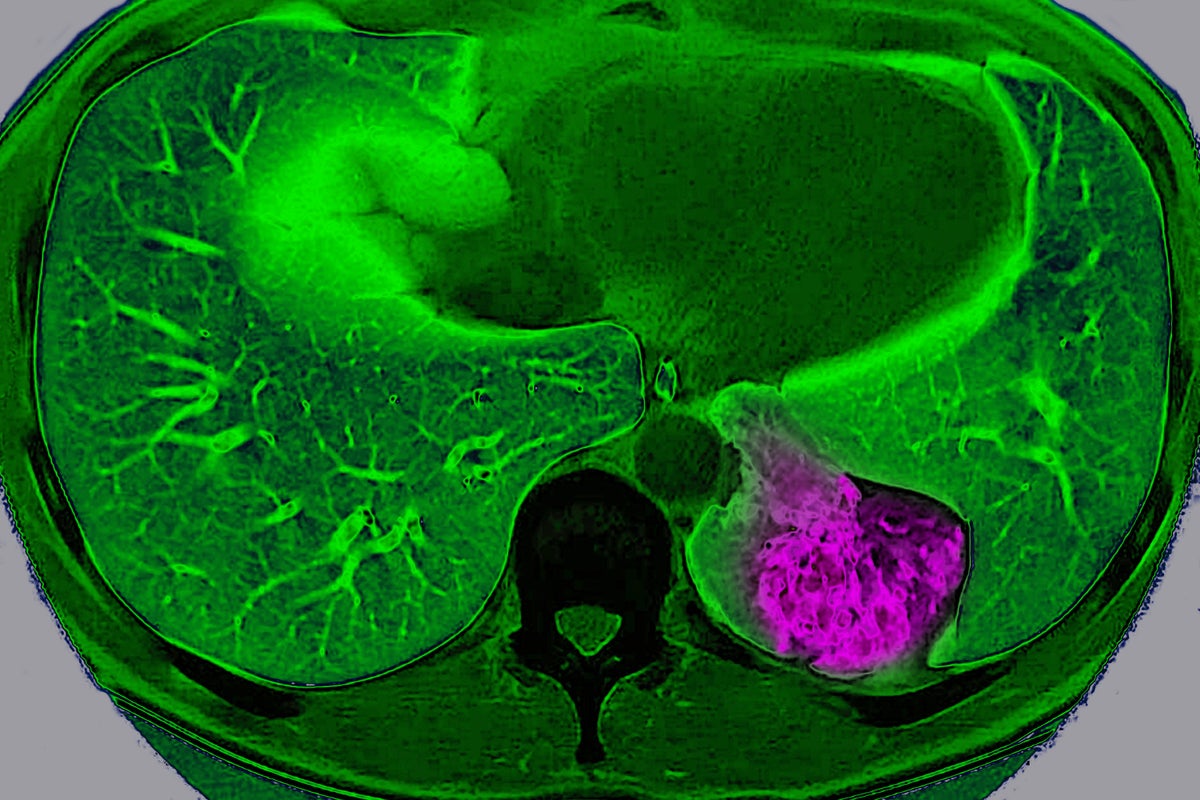
Lung cancer on the left pulmonary lobe, seen on a radial section MRI scan of the chest.
BSIP/Universal Images Group via Getty Images
For years, scientists have viewed cancer as a localized glitch in which cells refuse to stop dividing. But a new study suggests that, in certain organs, tumors actively communicate with the brain to trick it into protecting them.
Scientists have long known that nerves grow into some tumors and that tumors containing lots of nerves usually lead to a worse prognosis. But they didn’t know exactly why. “Prior to our study, most of the focus has been this local interaction between the nerve [endings] and the tumor,” says Chengcheng Jin, an assistant professor of cancer biology at the University of Pennsylvania and a co-author of the study, which was published today in Nature.
Jin and her colleagues discovered that lung cancer tumors in mice can use these nerve endings to communicate way beyond their close vicinity and send signals to the brain through a complex neuroimmune circuit. They also confirmed the circuit exists in humans.
On supporting science journalism
If you’re enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Setting up this circuit starts with a process called innervation, in which lung tumors wire themselves into the vagal nerves—the internal information highway that connects the vital organs to the brain. Within this highway, Jin’s team identified a specialized group of sensory neurons that communicate directly with the central nervous system. “Our study suggests that the tumor actually hijacks these existing pathways to promote itself,” explains Rui Chang, an associate professor of neuroscience at the Yale School of Medicine and a co-author of the study.
When a tumor develops, it employs vagal neurons to send signals screaming up to the nucleus of the solitary tract—the region in the brain stem that, under normal circumstances, keeps functions such as blood pressure, heart rate or digestion in check. The signal sent by the tumor exploits this system, much like malicious code used by a hacker.
Instead of recognizing the tumor as an invader that needs to be destroyed, the brain processes the signal and activates the sympathetic nervous system, mainly known as the driver of the fight-or-flight response. This sympathetic surge is caused by the release of noradrenaline, which, in the context of cancer, has catastrophic consequences.
The noradrenaline is released directly in the tumor’s immediate neighborhood, where it attaches to macrophages—the frontline cells of the immune system that identify, eat and destroy threats. The macrophages are covered in docking stations called β2 adrenergic receptors, which normally tell the cells when to be aggressive and when to “chill,” preventing the immune system from destroying healthy cells. When the noradrenaline released by the brain-controlled nerves binds to these receptors, it effectively reprograms the macrophages to switch sides.
In this suppressed state, they start releasing chemical signals that act as a “do not disturb” sign for the rest of the immune system. This neutralizes one of the body’s most effective weapons: T cells, the specialized assassins that physically kill tumor cells. Because the brain has ordered the macrophages to create an immunosuppressive shield, the T cells lose their energy, stop multiplying and fail to recognize the cancer as a threat.
“The authors characterized an entire bidirectional tumor-neural pathway that promotes tumor growth, with huge relevance to human health,” says Catherine Dulac, a professor of molecular and cellular biology at Harvard University, who was not involved in the study.
Jin and her team also looked for ways to stop tumors from talking to the brain. By mapping this loop from the lung to the brain and back again, the researchers identified several new places where they could “cut the wire.” The study showed that blocking any part of the brain-tumor circuit reawakened the immune system.
“Obviously, the perspective for application to cancer treatment is extremely promising,” Dulac says. Jin and Chang say we’re still rather far away from translating their findings into therapeutic strategies, however.
“What we are talking about is going from a mouse model to human. I think there’s still a long way to go,” Chang says.
It’s Time to Stand Up for Science
If you enjoyed this article, I’d like to ask for your support. Scientific American has served as an advocate for science and industry for 180 years, and right now may be the most critical moment in that two-century history.
I’ve been a Scientific American subscriber since I was 12 years old, and it helped shape the way I look at the world. SciAm always educates and delights me, and inspires a sense of awe for our vast, beautiful universe. I hope it does that for you, too.
If you subscribe to Scientific American, you help ensure that our coverage is centered on meaningful research and discovery; that we have the resources to report on the decisions that threaten labs across the U.S.; and that we support both budding and working scientists at a time when the value of science itself too often goes unrecognized.
In return, you get essential news, captivating podcasts, brilliant infographics, can’t-miss newsletters, must-watch videos, challenging games, and the science world’s best writing and reporting. You can even gift someone a subscription.
There has never been a more important time for us to stand up and show why science matters. I hope you’ll support us in that mission.