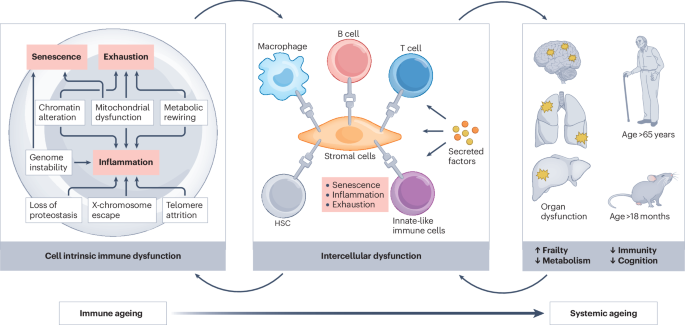
Sender, R. et al. The total mass, number, and distribution of immune cells in the human body. Proc. Natl Acad. Sci. USA 120, e2308511120 (2023).
Walford, R. L. The immunologic theory of aging. Gerontologist 4, 195–197 (1964).
Effros, R. B. Roy Walford and the immunologic theory of aging. Immun. Ageing 2, 7 (2005).
Yousefzadeh, M. J. et al. An aged immune system drives senescence and ageing of solid organs. Nature 594, 100–105 (2021). This article highlights a causal link between immune ageing and systemic ageing by utilizing an immune cell-specific DNA damage mouse model (Vav-iCre+/−;Ercc1−/fl).
Svensson, E. C. et al. TET2-driven clonal hematopoiesis and response to canakinumab: an exploratory analysis of the CANTOS randomized clinical trial. JAMA Cardiol. 7, 521–528 (2022).
Desdín-Micó, G. et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 368, 1371–1376 (2020). This article demonstrates that mitochondria dysfunction in T cells has a critical role in driving multiple age-related pathologies, potentially through reduced NAD+ levels.
Iborra-Pernichi, M. et al. Defective mitochondria remodelling in B cells leads to an aged immune response. Nat. Commun. 15, 2569 (2024).
Liu, Z. et al. Immunosenescence: molecular mechanisms and diseases. Signal. Transduct. Target. Ther. 8, 200 (2023).
Nikolich-Žugich, J., Li, G., Uhrlaub, J. L., Renkema, K. R. & Smithey, M. J. Age-related changes in CD8 T cell homeostasis and immunity to infection. Semin. Immunol. 24, 356–364 (2012).
Mogilenko, D. A., Shchukina, I. & Artyomov, M. N. Immune ageing at single-cell resolution. Nat. Rev. Immunol. 22, 484–498 (2022).
Sayed, N. et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat. Aging 1, 598–615 (2021).
Alpert, A. et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 25, 487–495 (2019).
Li, W. et al. Single-cell immune aging clocks reveal inter-individual heterogeneity during infection and vaccination. Nat. Aging 5, 607–621 (2025).
Ding, Y. et al. Comprehensive human proteome profiles across a 50-year lifespan reveal aging trajectories and signatures. Cell 188, 5763–5784.e26 (2025). This article provides a comprehensive overview and resource of proteome profiles across human organs during ageing.
Park, M. D. et al. Hematopoietic aging promotes cancer by fueling IL-1α-driven emergency myelopoiesis. Science 386, eadn0327 (2024). This article highlights how IL-1α-driven myelopoiesis contributes to the failure to control lung cancer progression with age.
Rettkowski, J. et al. Modulation of bone marrow haematopoietic stem cell activity as a therapeutic strategy after myocardial infarction: a preclinical study. Nat. Cell Biol. 27, 591–604 (2025).
Wang, Y. et al. Reducing functionally defective old HSCs alleviates aging-related phenotypes in old recipient mice. Cell Res. 35, 45–58 (2025).
Ross, J. B. et al. Depleting myeloid-biased haematopoietic stem cells rejuvenates aged immunity. Nature 628, 162–170 (2024). This article identifies a CD150high HSC population that drives myeloid-biased output from bone marrow, compromising the quality of adaptive immune response in ageing.
Morganti, C. & Ito, K. Mitochondrial contributions to hematopoietic stem cell aging. Int. J. Mol. Sci. 22, 11117 (2021).
Brown, K. et al. SIRT3 reverses aging-associated degeneration. Cell Rep. 3, 319–327 (2013).
Girotra, M. et al. Induction of mitochondrial recycling reverts age-associated decline of the hematopoietic and immune systems. Nat. Aging 3, 1057–1066 (2023).
Dorshkind, K., Höfer, T., Montecino-Rodriguez, E., Pioli, P. D. & Rodewald, H. R. Do haematopoietic stem cells age? Nat. Rev. Immunol. 20, 196–202 (2020).
Elias, H. K. et al. Kitlo hematopoietic stem cells exhibit distinct lymphoid-primed chromatin landscapes that enhance thymic reconstitution. Nat. Commun. 16, 6170 (2025).
Sun, N. et al. Clusterin drives myeloid bias in aged hematopoietic stem cells by regulating mitochondrial function. Nat. Aging 5, 1510–1527 (2025).
Fuster, J. J. et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847 (2017).
Jaiswal, S. & Ebert, B. L. Clonal hematopoiesis in human aging and disease. Science 366, eaan4673 (2019).
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
Wang, H. et al. Clonal hematopoiesis driven by mutated DNMT3A promotes inflammatory bone loss. Cell 187, 3690–3711.e19 (2024). This article reports the detrimental effect of DNMT3A-driven clonal haematopoiesis on periodontitis and demonstrates that rapapmycin can reverse periodontitis-associated pathologies.
Garagnani, P. et al. Whole-genome sequencing analysis of semi-supercentenarians. eLife 10, e57849 (2021).
Bouzid, H. et al. Clonal hematopoiesis is associated with protection from Alzheimer’s disease. Nat. Med. 29, 1662–1670 (2023). This article describes a negative association between clonal haematopoiesis and Alzheimer disease, potentially mediated by alterations in the microglial pool.
Matatall, K. A. et al. TET2-mutant myeloid cells mitigate Alzheimer’s disease progression via CNS infiltration and enhanced phagocytosis in mice. Cell Stem Cell 32, 1285–1298.e8 (2025).
Kapadia, C. D. et al. Clonal dynamics and somatic evolution of haematopoiesis in mouse. Nature 641, 681–689 (2025).
Ambrosi, T. H. et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 20, 771–784.e6 (2017).
Toghani, D. et al. Niche-derived Semaphorin 4A safeguards functional identity of myeloid-biased hematopoietic stem cells. Nat. Aging 5, 558–575 (2025).
Pioli, P. D., Casero, D., Montecino-Rodriguez, E., Morrison, S. L. & Dorshkind, K. Plasma cells are obligate effectors of enhanced myelopoiesis in aging bone marrow. Immunity 51, 351–366.e6 (2019).
Ambrosi, T. H. et al. Aged skeletal stem cells generate an inflammatory degenerative niche. Nature 597, 256–262 (2021).
Mittelbrunn, M. & Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 22, 687–698 (2021).
Han, S., Georgiev, P., Ringel, A. E., Sharpe, A. H. & Haigis, M. C. Age-associated remodeling of T cell immunity and metabolism. Cell Metab. 35, 36–55 (2023).
Goronzy, J. J. & Weyand, C. M. Mechanisms underlying T cell ageing. Nat. Rev. Immunol. 19, 573–583 (2019).
Frasca, D., Diaz, A., Romero, M., Garcia, D. & Blomberg, B. B. B cell immunosenescence. Annu. Rev. Cell Dev. Biol. 36, 551–574 (2020).
Valentino, T. R. et al. The role of autoantibodies in bridging obesity, aging, and immunosenescence. Immun. Ageing 21, 85 (2024).
Cancro, M. P. Age-associated B cells. Annu. Rev. Immunol. 38, 315–340 (2020).
Zhang, H. et al. Aging-associated HELIOS deficiency in naive CD4. Nat. Immunol. 24, 96–109 (2023).
Castro, J. P. et al. Age-associated clonal B cells drive B cell lymphoma in mice. Nat. Aging 4, 1403–1417 (2024).
Ovadya, Y. et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 9, 5435 (2018).
Wang, T. W. et al. Blocking PD-L1-PD-1 improves senescence surveillance and ageing phenotypes. Nature 611, 358–364 (2022). This article demonstrates that the age-related decline in immunosurveilance promotes the accumulation of senescent cells and highlights the therapeutic potential of immunotherapy to restore immune function and alleviate systemic ageing.
Dahlquist, K. J. V. et al. PD1 blockade improves survival and CD8. Nat. Aging 4, 915–925 (2024).
Elyahu, Y. et al. Aging promotes reorganization of the CD4 T cell landscape toward extreme regulatory and effector phenotypes. Sci. Adv. 5, eaaw8330 (2019).
Zukowski, E. et al. STAT3 modulates CD4. Aging Cell 22, e13996 (2023).
Callender, L. A. et al. Mitochondrial mass governs the extent of human T cell senescence. Aging Cell 19, e13067 (2020).
Zhang, H. et al. PREX1 improves homeostatic proliferation to maintain a naive CD4+ T cell compartment in older age. JCI Insight 9, e172848 (2024).
Jin, J. et al. CISH impairs lysosomal function in activated T cells resulting in mitochondrial DNA release and inflammaging. Nat. Aging 3, 600–616 (2023).
Headley, C. A. et al. Extracellular delivery of functional mitochondria rescues the dysfunction of CD4. Adv. Sci. 11, e2303664 (2024).
Quinn, K. M. et al. Age-related decline in primary CD8. Cell Rep. 23, 3512–3524 (2018).
Quinn, K. M., Vicencio, D. M. & La Gruta, N. L. The paradox of aging: aging-related shifts in T cell function and metabolism. Semin. Immunol. 70, 101834 (2023).
Terekhova, M. et al. Single-cell atlas of healthy human blood unveils age-related loss of NKG2C. Immunity 57, 188–192 (2024).
Lan, F. et al. GZMK-expressing CD8. Nature 638, 490–498 (2025).
Pei, S. et al. Age-related decline in CD8. Nat. Aging 4, 1828–1844 (2024).
Camell, C. D. et al. Aging induces an Nlrp3 inflammasome-dependent expansion of adipose B cells that impairs metabolic homeostasis. Cell Metab. 30, 1024–1039.e6 (2019).
Souyris, M. et al. escapes X chromosome inactivation in immune cells. Sci. Immunol. 3, eaap8855 (2018).
Luo, Y. et al. Single-cell genomics identifies distinct B1 cell developmental pathways and reveals aging-related changes in the B-cell receptor repertoire. Cell Biosci. 12, 57 (2022).
Marín-Aguilar, F. et al. NLRP3 inflammasome inhibition by MCC950 in aged mice improves health via enhanced autophagy and PPARα activity. J. Gerontol. A Biol. Sci. Med. Sci 75, 1457–1464 (2020).
Oishi, Y. & Manabe, I. Macrophages in age-related chronic inflammatory diseases. npj Aging Mech. Dis. 2, 16018 (2016).
Millet, A., Ledo, J. H. & Tavazoie, S. F. An exhausted-like microglial population accumulates in aged and APOE4 genotype Alzheimer’s brains. Immunity 57, 153–170.e6 (2024).
Hu, H. et al. Defective efferocytosis by aged macrophages promotes STING signaling mediated inflammatory liver injury. Cell Death Discov. 9, 236 (2023).
Blacher, E. et al. Aging disrupts circadian gene regulation and function in macrophages. Nat. Immunol. 23, 229–236 (2022).
Ryu, S. et al. The matricellular protein SPARC induces inflammatory interferon-response in macrophages during aging. Immunity 55, 1609–1626.e7 (2022).
Hou, J. et al. Aged bone marrow macrophages drive systemic aging and age-related dysfunction via extracellular vesicle-mediated induction of paracrine senescence. Nat. Aging 4, 1562–1581 (2024). This article introduces a critical concept of paracrine senescence driven by aged macrophages, highlighting their contribution to systemic ageing.
Nogueira-Recalde, U. et al. Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. EBioMedicine 45, 588–605 (2019). This article establishes the concept of paracrine senescence driven by immunoglobulin, contributing to systemic ageing.
Ma, S. et al. Spatial transcriptomic landscape unveils immunoglobin-associated senescence as a hallmark of aging. Cell 187, 7025–7044.e34 (2024).
Sawaki, D. et al. Osteopontin promotes age-related adipose tissue remodeling through senescence-associated macrophage dysfunction. JCI Insight 8, e145811 (2023).
Sawaki, D. et al. Visceral adipose tissue drives cardiac aging through modulation of fibroblast senescence by osteopontin production. Circulation 138, 809–822 (2018).
Zhou, Z. et al. Type 2 cytokine signaling in macrophages protects from cellular senescence and organismal aging. Immunity 57, 513–527.e6 (2024).
Zhang, D. et al. Neutrophil ageing is regulated by the microbiome. Nature 525, 528–532 (2015).
Van Avondt, K. et al. Neutrophils in aging and aging-related pathologies. Immunol. Rev. 314, 357–375 (2023).
Barkaway, A. et al. Age-related changes in the local milieu of inflamed tissues cause aberrant neutrophil trafficking and subsequent remote organ damage. Immunity 54, 1494–1510.e7 (2021). This article highlights that neutrophils, which are relatively understudied innate immune cells in the context of ageing, drive remote tissue inflammation via their re-entry into the circulation.
Lu, R. J. et al. Multi-omic profiling of primary mouse neutrophils predicts a pattern of sex and age-related functional regulation. Nat. Aging 1, 715–733 (2021).
Brauning, A. et al. Aging of the immune system: focus on natural killer cells phenotype and functions. Cells 11, 1017 (2022).
Shehata, H. M., Hoebe, K. & Chougnet, C. A. The aged nonhematopoietic environment impairs natural killer cell maturation and function. Aging Cell 14, 191–199 (2015).
Almeida-Oliveira, A. et al. Age-related changes in natural killer cell receptors from childhood through old age. Hum. Immunol. 72, 319–329 (2011).
Gounder, S. S. et al. Effect of aging on NK cell population and their proliferation at ex vivo culture condition. Anal. Cell Pathol. 2018, 7871814 (2018).
D’Souza, S. S. et al. Compartmentalized effects of aging on group 2 innate lymphoid cell development and function. Aging Cell 18, e13019 (2019).
Goldberg, E. L. et al. IL-33 causes thermogenic failure in aging by expanding dysfunctional adipose ILC2. Cell Metab. 33, 2277–2287.e5 (2021).
Fung, I. T. H. et al. Activation of group 2 innate lymphoid cells alleviates aging-associated cognitive decline. J. Exp. Med. 217, e20190915 (2020).
Gray, J. I. et al. Human γδ T cells in diverse tissues exhibit site-specific maturation dynamics across the life span. Sci. Immunol. 9, eadn3954 (2024).
Bruno, M. E. C. et al. Accumulation of γδ T cells in visceral fat with aging promotes chronic inflammation. Geroscience 44, 1761–1778 (2022).
Faust, H. J. et al. IL-17 and immunologically induced senescence regulate response to injury in osteoarthritis. J. Clin. Invest. 130, 5493–5507 (2020).
Mukherjee, S. et al. Mechanisms of γδ T cell accumulation in visceral adipose tissue with aging. Front. Aging 4, 1258836 (2023).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Kroemer, G. et al. From geroscience to precision geromedicine: understanding and managing aging. Cell 188, 2043–2062 (2025). Recent developments in geroscience are discussed, and extracellular matrix alterations are introduced as a new hallmark of ageing in this paper.
Pieren, D. K. J. et al. Compromised DNA repair promotes the accumulation of regulatory T cells with an aging-related phenotype and responsiveness. Front. Aging 2, 667193 (2021).
Rossiello, F., Jurk, D., Passos, J. F. & d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 24, 135–147 (2022).
Najarro, K. et al. Telomere length as an indicator of the robustness of B- and T-cell response to influenza in older adults. J. Infect. Dis. 212, 1261–1269 (2015).
Kang, Y. et al. Telomere dysfunction disturbs macrophage mitochondrial metabolism and the NLRP3 inflammasome through the PGC-1α/TNFAIP3 axis. Cell Rep. 22, 3493–3506 (2018).
Vukmanovic-Stejic, M. et al. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J. Clin. Invest. 116, 2423–2433 (2006).
Lanna, A. et al. An intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory. Nat. Cell Biol. 24, 1461–1474 (2022). This article discusses the potential implications of impaired intercellular telomere transfer in driving immune and systemic ageing.
Amorim, J. A. et al. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 18, 243–258 (2022).
Zong, Y. et al. Mitochondrial dysfunction: mechanisms and advances in therapy. Signal. Transduct. Target. Ther. 9, 124 (2024).
Wculek, S. K. et al. Oxidative phosphorylation selectively orchestrates tissue macrophage homeostasis. Immunity 56, 516–530.e9 (2023).
Seegren, P. V. et al. Reduced mitochondrial calcium uptake in macrophages is a major driver of inflammaging. Nat. Aging 3, 796–812 (2023).
Victorelli, S. et al. Apoptotic stress causes mtDNA release during senescence and drives the SASP. Nature 622, 627–636 (2023).
Wang, K. et al. Epigenetic regulation of aging: implications for interventions of aging and diseases. Signal. Transduct. Target. Ther. 7, 374 (2022).
Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 14, R115 (2013).
Kusters, C. D. J. & Horvath, S. Quantification of epigenetic aging in public health. Annu. Rev. Public Health 46, 91–110 (2025).
Márquez, E. J. et al. Sexual-dimorphism in human immune system aging. Nat. Commun. 11, 751 (2020). This article highlights age-related epigenetic alterations in immune cells by characterizing peripheral blood mononuclear cells from 172 individuals and provides a resource to explore the impact of age and sex on immune phenotype.
Shchukina, I. et al. Enhanced epigenetic profiling of classical human monocytes reveals a specific signature of healthy aging in the DNA methylome. Nat. Aging 1, 124–141 (2021).
Sano, S. et al. Hematopoietic loss of Y chromosome leads to cardiac fibrosis and heart failure mortality. Science 377, 292–297 (2022).
Horitani, K. et al. Disruption of the Uty epigenetic regulator locus in hematopoietic cells phenocopies the profibrotic attributes of Y chromosome loss in heart failure. Nat. Cardiovasc. Res. 3, 343–355 (2024).
Karakaslar, E. O. et al. Transcriptional activation of Jun and Fos members of the AP-1 complex is a conserved signature of immune aging that contributes to inflammaging. Aging Cell 22, e13792 (2023).
Jang, I. H. et al. GDF3 promotes adipose tissue macrophage-mediated inflammation via altered chromatin accessibility during aging. Nat. Aging 6, 127–142 (2026).
Moss, C. E. et al. Aging-related defects in macrophage function are driven by MYC and USF1 transcriptional programs. Cell Rep. 43, 114073 (2024).
Arata, Y. et al. Defective induction of the proteasome associated with T-cell receptor signaling underlies T-cell senescence. Genes Cell 24, 801–813 (2019).
Aman, Y. et al. Autophagy in healthy aging and disease. Nat. Aging 1, 634–650 (2021).
Zhang, H. et al. Polyamines control eIF5A hypusination, TFEB translation, and autophagy to reverse B cell senescence. Mol. Cell 76, 110–125.e9 (2019).
Alsaleh, G. et al. Autophagy in T cells from aged donors is maintained by spermidine and correlates with function and vaccine responses. eLife 9, e57950 (2020).
Dellorusso, P. V. et al. Autophagy counters inflammation-driven glycolytic impairment in aging hematopoietic stem cells. Cell Stem Cell 31, 1020–1037.e9 (2024).
Consortium, T. M. et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372 (2018). This article provides a comprehensive single-cell level resource detailling age-related changes in immune cells across 20 mouse organs.
Camell, C. D. et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550, 119–123 (2017).
Wang, Y. et al. Integrating single-cell RNA and T cell/B cell receptor sequencing with mass cytometry reveals dynamic trajectories of human peripheral immune cells from birth to old age. Nat. Immunol. 26, 308–322 (2025).
Yadav, S., Deepika & Maurya, P. K. A systematic review of red blood cells biomarkers in human aging. J. Gerontol. A Biol. Sci. Med. Sci. 79, glae004 (2024).
Ningtyas, D. C. et al. Platelets mediate the clearance of senescent red blood cells by forming prophagocytic platelet-cell complexes. Blood 143, 535–547 (2024).
Sage, P. T., Tan, C. L., Freeman, G. J., Haigis, M. & Sharpe, A. H. Defective TFH cell function and increased TFR cells contribute to defective antibody production in aging. Cell Rep. 12, 163–171 (2015).
Stebegg, M. et al. Rejuvenating conventional dendritic cells and T follicular helper cell formation after vaccination. eLife 9, e52473 (2020).
Lee, J. L. et al. B cell-intrinsic changes with age do not impact antibody-secreting cell formation but delay B cell participation in the germinal centre reaction. Aging Cell 21, e13692 (2022).
Ou, M. Y., Zhang, H., Tan, P. C., Zhou, S. B. & Li, Q. F. Adipose tissue aging: mechanisms and therapeutic implications. Cell Death Dis. 13, 300 (2022).
Carey, A. et al. B-cell interleukin 1 receptor 1 (IL1R1) modulates the female adipose tissue immune microenvironment during aging. J. Leukoc. Biol. 117, qiae219 (2025).
Carey, A. et al. Age-associated accumulation of B cells promotes macrophage inflammation and inhibits lipolysis in adipose tissue during sepsis. Cell Rep. 43, 113967 (2024).
Yu, L. et al. IgG is an aging factor that drives adipose tissue fibrosis and metabolic decline. Cell Metab. 36, 793–807.e5 (2024).
Chini, C. C. S. et al. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD. Nat. Metab. 2, 1284–1304 (2020).
Covarrubias, A. J. et al. Senescent cells promote tissue NAD. Nat. Metab. 2, 1265–1283 (2020).
Camacho-Pereira, J. et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 23, 1127–1139 (2016).
Kalathookunnel Antony, A., Lian, Z. & Wu, H. T cells in adipose tissue in aging. Front. Immunol. 9, 2945 (2018).
Brigger, D. et al. Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age. Nat. Metab. 2, 688–702 (2020).
Feng, X. et al. Senescent immune cells accumulation promotes brown adipose tissue dysfunction during aging. Nat. Commun. 14, 3208 (2023).
Stahl, E. C. et al. Inflammation and ectopic fat deposition in the aging murine liver is influenced by CCR2. Am. J. Pathol. 190, 372–387 (2020).
Lagnado, A. et al. Neutrophils induce paracrine telomere dysfunction and senescence in ROS-dependent manner. EMBO J. 40, e106048 (2021).
Du, K. et al. Aging promotes metabolic dysfunction-associated steatotic liver disease by inducing ferroptotic stress. Nat. Aging 4, 949–968 (2024).
Solá, P. et al. Targeting lymphoid-derived IL-17 signaling to delay skin aging. Nat. Aging 3, 688–704 (2023).
Han, J. et al. Age-associated senescent – T cell signaling promotes type 3 immunity that inhibits the biomaterial regenerative response. Adv. Mater. 36, e2310476 (2024).
Allen, W. E., Blosser, T. R., Sullivan, Z. A., Dulac, C. & Zhuang, X. Molecular and spatial signatures of mouse brain aging at single-cell resolution. Cell 186, 194–208.e18 (2023).
Gulen, M. F. et al. cGAS-STING drives ageing-related inflammation and neurodegeneration. Nature 620, 374–380 (2023). This article demonstrates the potential of immunotheraphy through blockade of inflammatory pathways to mitigate age-related pathologies.
Kang, S. et al. Microglia undergo sex-dimorphic transcriptional and metabolic rewiring during aging. J. Neuroinflammation 21, 150 (2024).
Zhang, X. et al. Rejuvenation of the aged brain immune cell landscape in mice through p16-positive senescent cell clearance. Nat. Commun. 13, 5671 (2022).
Kang, L. et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat. Commun. 11, 2488 (2020).
Zenaro, E. et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 21, 880–886 (2015).
Gullotta, G. S. et al. Age-induced alterations of granulopoiesis generate atypical neutrophils that aggravate stroke pathology. Nat. Immunol. 24, 925–940 (2023).
Dulken, B. W. et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 571, 205–210 (2019).
de la Fuente, A. G. et al. Ageing impairs the regenerative capacity of regulatory T cells in mouse central nervous system remyelination. Nat. Commun. 15, 1870 (2024).
Jin, W. N. et al. Neuroblast senescence in the aged brain augments natural killer cell cytotoxicity leading to impaired neurogenesis and cognition. Nat. Neurosci. 24, 61–73 (2021).
Misharin, A. V. et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 214, 2387–2404 (2017).
Farhat, A. et al. An aging bone marrow exacerbates lung fibrosis by fueling profibrotic macrophage persistence. Sci. Immunol. 10, eadk5041 (2025).
McQuattie-Pimentel, A. C. et al. The lung microenvironment shapes a dysfunctional response of alveolar macrophages in aging. J. Clin. Invest. 131, e140299 (2021).
Prieto, L. I. et al. Senescent alveolar macrophages promote early-stage lung tumorigenesis. Cancer Cell 41, 1261–1275.e6 (2023).
Rasa, S. M. M. et al. Inflammaging is driven by upregulation of innate immune receptors and systemic interferon signaling and is ameliorated by dietary restriction. Cell Rep. 39, 111017 (2022).
Monzó, C. et al. Dietary restriction mitigates the age-associated decline in mouse B cell receptor repertoire diversity. Cell Rep. 42, 112722 (2023).
Tao, S. et al. Long-term mid-onset dietary restriction rejuvenates hematopoietic stem cells and improves regeneration capacity of total bone marrow from aged mice. Aging Cell 19, e13241 (2020).
Papp, G. et al. Regular exercise may restore certain age-related alterations of adaptive immunity and rebalance immune regulation. Front. Immunol. 12, 639308 (2021).
Di Francesco, A. et al. Dietary restriction impacts health and lifespan of genetically diverse mice. Nature 634, 684–692 (2024).
Kraus, W. E. et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 7, 673–683 (2019). This article describes a randomized calorie restriction clinical trial that served as the basis for identifying multiple molecular mechanisms governing healthspan.
Spadaro, O. et al. Caloric restriction in humans reveals immunometabolic regulators of health span. Science 375, 671–677 (2022).
Youm, Y. H. et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 21, 263–269 (2015).
Ryu, S. et al. Ketogenic diet restrains aging-induced exacerbation of coronavirus infection in mice. eLife 10, e66522 (2021).
Goldberg, E. L. et al. β-Hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep. 18, 2077–2087 (2017).
Lee, A. H. & Dixit, V. D. Dietary regulation of immunity. Immunity 53, 510–523 (2020).
Newman, J. C. et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 26, 547–557.e8 (2017).
Conway, J. et al. Age-related loss of intestinal barrier integrity plays an integral role in thymic involution and T cell ageing. Aging Cell 24, e14401 (2025).
Thevaranjan, N. et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 21, 455–466.e4 (2017).
Bodogai, M. et al. Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci. Transl. Med. 10, eaat4271 (2018).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020). This article demonstrates that immunotheraphy using CAR T cells to target uPAR-expressing senescent cells can mitigate age-related pathologies.
Amor, C. et al. Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction. Nat. Aging 7, 336–349 (2024).
Iltis, C. et al. A ganglioside-based immune checkpoint enables senescent cells to evade immunosurveillance during aging. Nat. Aging 5, 219–236 (2025).
Wang, T. W. & Nakanishi, M. Immune surveillance of senescence: potential application to age-related diseases. Trends Cell Biol. 35, 248–257 (2025).
Zhu, Y. et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-X. Aging 9, 955–963 (2017).
Yousefzadeh, M. J. et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36, 18–28 (2018).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018). This article demonstrates the potential of senotherapeutics approaches to improve immune function and extend healthspan.
Zhu, Y. et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 (2015).
Camell, C. D. et al. Senolytics reduce coronavirus-related mortality in old mice. Science 373, eabe4832 (2021).
Farr, J. N. et al. Local senolysis in aged mice only partially replicates the benefits of systemic senolysis. J. Clin. Invest. 133, e162519 (2023).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Ocampo, A. et al. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 167, 1719–1733.e2 (2016).
Lu, Y. et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 588, 124–129 (2020).
Sarkar, T. J. et al. Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells. Nat. Commun. 11, 1545 (2020).
Yang, J. H. et al. Chemically induced reprogramming to reverse cellular aging. Aging 15, 5966–5989 (2023).
Montecino-Rodriguez, E., Berent-Maoz, B. & Dorshkind, K. Causes, consequences, and reversal of immune system aging. J. Clin. Invest. 123, 958–965 (2013).
Duggal, N. A. Reversing the immune ageing clock: lifestyle modifications and pharmacological interventions. Biogerontology 19, 481–496 (2018).
Frisch, B. J. et al. Aged marrow macrophages expand platelet-biased hematopoietic stem cells via Interleukin1B. JCI Insight 5, e124213 (2019).
Sutherland, T. E., Dyer, D. P. & Allen, J. E. The extracellular matrix and the immune system: a mutually dependent relationship. Science 379, eabp8964 (2023). This article discusses the reciprocal interactions between the extracellular matrix and the immune system, highlighting their potential implications in immune and systemic ageing.
Harper, E. I. & Weeraratna, A. T. A wrinkle in TIME: how changes in the aging ECM drive the remodeling of the tumor immune microenvironment. Cancer Discov. 13, 1973–1981 (2023).
Gubbels Bupp, M. R., Potluri, T., Fink, A. L. & Klein, S. L. The confluence of sex hormones and aging on immunity. Front. Immunol. 9, 1269 (2018).
Fairweather, D., Beetler, D. J., McCabe, E. J. & Lieberman, S. M. Mechanisms underlying sex differences in autoimmunity. J. Clin. Invest. 134, e180076 (2024).
Dunn, S. E., Perry, W. A. & Klein, S. L. Mechanisms and consequences of sex differences in immune responses. Nat. Rev. Nephrol. 20, 37–55 (2024).
Zheng, Y., Liu, Q., Goronzy, J. J. & Weyand, C. M. Immune aging — a mechanism in autoimmune disease. Semin. Immunol. 69, 101814 (2023).
Liu, Q., Zheng, Y., Goronzy, J. J. & Weyand, C. M. T cell aging as a risk factor for autoimmunity. J. Autoimmun. 137, 102947 (2023).
Hagen, S. H. et al. Heterogeneous escape from X chromosome inactivation results in sex differences in type I IFN responses at the single human pDC level. Cell Rep. 33, 108485 (2020).
Abdulai-Saiku, S. et al. The maternal X chromosome affects cognition and brain ageing in female mice. Nature 638, 152–159 (2025).
Arai, Y. et al. Hematopoietic loss of Y chromosome activates immune checkpoints and contributes to impaired senescent cell clearance and renal disease. Sci. Transl. Med. 17, eadv4071 (2025).
Landry, D. A. et al. Metformin prevents age-associated ovarian fibrosis by modulating the immune landscape in female mice. Sci. Adv. 8, eabq1475 (2022).
Li, Y. et al. Single-cell analysis reveals alternations between the aged and young mice prostates. Biomark Res. 12, 117 (2024).
Benedusi, V. et al. Ovariectomy shortens the life span of female mice. Oncotarget 6, 10801–10811 (2015).