RICHMOND, Va. (InvestigateTV) — Sadie Johnson was in sixth grade when she started getting chemical treatments to straighten her hair.
For the next four decades, the Virginia resident endured scalp burns and sores every two weeks.
“In the beginning, it was awful. Scalp would burn and hair would have miniature sores. I thought that it was just part of the process,” Johnson said. “Ten minutes. And she hadn’t finished perming the whole head then. By the time she finished perming the whole hair, my head was aflame.”
Johnson’s experience represents that of countless Black women who felt pressured to conform to imposed beauty standards in the 19th century through painful hair chemical treatments called “relaxers” sometimes referred as “perms” in the Black community.
 On the left, Sadie Johnson now rocks her natural hair. The photo on the right, Sadie is in pink wearing her hair chemically relaxed; this was a photo of her in high school.(Sadie Johnson)
On the left, Sadie Johnson now rocks her natural hair. The photo on the right, Sadie is in pink wearing her hair chemically relaxed; this was a photo of her in high school.(Sadie Johnson)
Those relaxers were intended to help keep coarse hair straight for weeks and help assimilate with society.
“I’m 68, so natural hair was considered to be ‘naughty’ hair,” Johnson said. “It was a standard beauty. Imposed standard of beauty, like your hair wasn’t good enough.”
The experience was so painful that Johnson described having to “suffer through to make it to the bowl” for rinsing. She said stylists would apply Vaseline to her scalp before the chemical treatment to provide some protection, but the burning persisted.
She also recalls the distinctive chemical smell during treatments, comparing it to formaldehyde used to preserve animals in science class.
“It smelled like…science class, and you had animals in jars that [had] formaldehyde or whatever,” Johnson said.
Johnson’s decades of chemical treatments eventually took their toll. Her hair began thinning at the top, and she developed medical issues that she said her dermatologist linked to harsh chemical use.
A national investigation reveals widespread concerns
But Johnson’s story is not unique. InvestigateTV found that popular hair straightening and smoothing products may contain chemicals known to cause cancer and other health issues.
Years of research and complaints show potential issues with products marketed to people with curly and textured hair, and many products with those chemicals are still on the market. The issue disproportionately impacts African American women.
InvestigateTV requested and obtained Food and Drug Administration documents showing hundreds of complaints from coast to coast since 2004, after studies began linking formaldehyde to cancer. The documents showcase hundreds of people reporting concerning outcomes they believe were tied to chemical hair straighteners.
Complaints included bald spots, hair loss, blisters, scars, and even hospitalizations after applying products to the entire head.
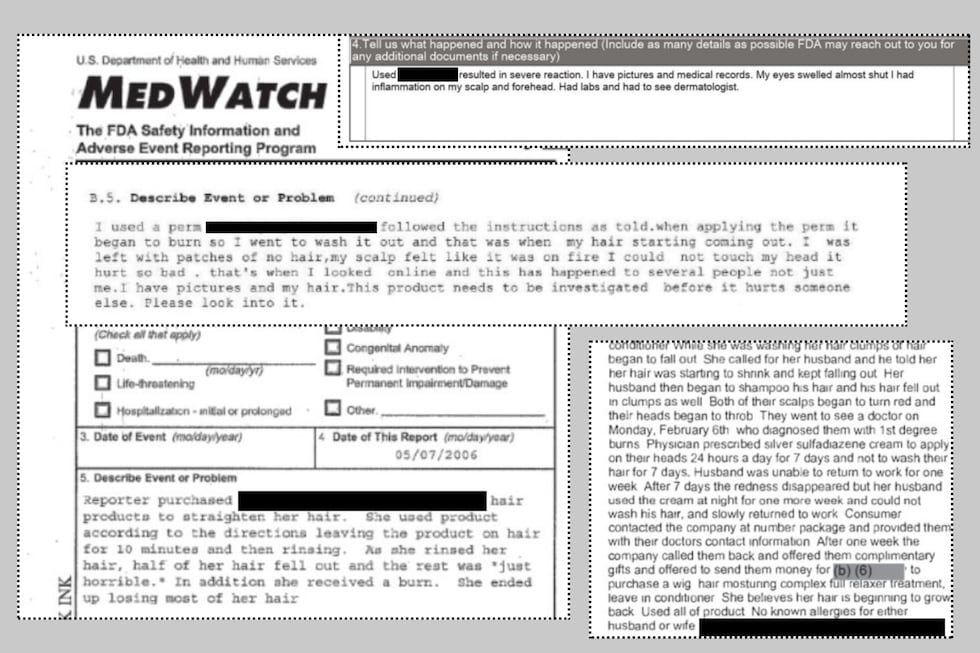 These are just some examples of complaints filed by consumers about their experiences using chemical hair relaxers.(Daniela Molina)
These are just some examples of complaints filed by consumers about their experiences using chemical hair relaxers.(Daniela Molina)
In one review, a 54-year-old woman experienced severe inflammation on her scalp and forehead, with her eyes swelling almost shut.
In another case, a consumer reported that she “used product according to the directions, leaving the product on her hair for 10 minutes and then rinsing. As she rinsed her hair, half of her hair fell out, and the rest was ‘just horrible.’ In addition to receiving a burn, she ended up losing most of her hair.”
Reports also documented chemical burns and cancer diagnoses.
The toxic chemical at the center: formaldehyde
The main chemical in question in many of these products is formaldehyde. It is a toxic gas that poses serious health risks to both consumers and salon workers linking to eye problems, respiratory issues and even certain types of cancer like ovarian, according to the National Cancer Institute.
“Formaldehyde is one of the active ingredients that makes that product work, and that makes your hair so shiny and slick and straight and stay that way for months at a time,” said Melanie Benesh, vice president of government affairs for Environmental Working Group, a nonprofit advocacy organization.
 Melanie Benesh with the Environmental Working Group hopes that the FDA will take action bannning formaldehyde soon.(InvestigateTV)
Melanie Benesh with the Environmental Working Group hopes that the FDA will take action bannning formaldehyde soon.(InvestigateTV)
For years, Benesh and the EWG have pushed the FDA to ban formaldehyde from being used by cosmetic companies.
“We have been working on this issue for over a decade,” Benesh said. “We got those reports related to formaldehyde. There were a lot of them, a lot of women who experienced health issues after getting these treatments or giving these treatments.”
Formaldehyde is classified as a known carcinogen by the EPA.
The FDA says formaldehyde and two related ingredients, formalin and methylene glycol, are some of the chemicals consumers should look for on labels.
“When the liquid form of formaldehyde, sometimes marketed as methylene glycol in these products, gets heated up, it can release a significant amount of formaldehyde gas,” Benesh said.
EWG put out a report in 2011 and filed its first citizen petition with the FDA that same year, asking them to consider banning formaldehyde in hair straightening products. They filed a second petition in 2021, asking again that the agency ban formaldehyde in hair products.
Despite national concern, the FDA has not banned the ingredients and has missed its fifth deadline to propose a ban on formaldehyde products.
Since 2024, the InvestigateTV team has reached out to the FDA for an interview or comment. After repeated efforts, a spokesperson from the FDA said:
FDA’s proposed rule, “Use of Formaldehyde and Formaldehyde-Releasing Chemicals as an Ingredient in Hair Smoothing Products or Hair Straightening Products” continues to remain a priority for the Agency.
The agency went on to say that the FDA may adjust publication dates for this and other proposed rules when appropriate, and several factors may impact its ability to finalize a rule including administration priorities, emerging public health, or other issues.
InvestigateTV also reached out to the companies mentioned in the FDA complaints about a variety of ingredients and products. Only L’Oréal responded to our questions. A spokesperson said:
“Our highest priority is the health and wellbeing of all our consumers. Our products are subject to a rigorous scientific evaluation of their safety by experts who also ensure that we strictly follow all regulations in every market in which we operate.
L’Oréal does not add formaldehyde as an ingredient in any of its products in any market in the world. We welcome and support the U.S. Food and Drug Administration’s (FDA) proposal across the entire beauty industry.”
A difficult but liberating transition
Frustrated by governmental inaction, some women have gone to the courts to take action against companies that use formaldehyde in their chemical hair straightening products.
Civil rights attorney Benjamin Crump has represented several in a class action lawsuit. Those suits are still pending.
 Attorney Ben Crump has filed hundreds of lawsuits on behalf of users of chemical hair straightening products. He says he wants to see the FDA act.(InvestigateTV)
Attorney Ben Crump has filed hundreds of lawsuits on behalf of users of chemical hair straightening products. He says he wants to see the FDA act.(InvestigateTV)
“These European standards of beauty that are injected on women of color, where they feel the need to try and get these hair relaxers to fit into corporate America, is detrimental to our health,” Crump said. “They’re so prevalent that they even sell them at gas stations. We want the FDA to act.”
As women wait for a change in regulation, many are ditching the relaxers altogether.
Johnson made that transition in 2015.
“I’ve always had a sense of pride. A sense of me, who I am. My mom used to say, ‘One day you’re going to wake up and you’re going to get it,’” Johnson said. “I know what she means now. I know what she means.”
Full FDA Statement:
FDA may adjust the anticipated publication date of this and other proposed rules when appropriate. Several factors may impact FDA’s ability to propose and finalize a rule, including, for example, Administration priorities, emerging public health issues, or other extenuating circumstances. FDA will provide periodic updates to the projected publication dates of upcoming rulemakings through the Unified Agenda, which is published semi-annually. The Unified Agenda is released by the Office of Management and Budget (OMB) and reports on regulatory actions under development that federal agencies plan to issue in the near and long term. You can find more information about rules and rulemaking at FDA Rules and Regulations and the Reginfo.gov site’s Frequently Asked Questions page.
ATTENTION: By clicking submit on the survey below, you agree to our terms and conditions regarding your information and image.
Copyright 2026 Gray Media Group, Inc. All Rights Reserved.