Chow, L. Q. M. Head and neck cancer. N. Engl. J. Med. 382, 60–72 (2020).
Johnson, D. E. et al. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 6, 92 (2020).
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Mody, M. D., Rocco, J. W., Yom, S. S., Haddad, R. I. & Saba, N. F. Head and neck cancer. Lancet 398, 2289–2299 (2021).
Barsouk, A., Aluru, J. S., Rawla, P., Saginala, K. & Barsouk, A. Epidemiology, risk factors, and prevention of head and neck squamous cell carcinoma. Med. Sci. 11, 42 (2023).
Thakral, A. et al. Smoking and alcohol by HPV status in head and neck cancer: a Mendelian randomization study. Nat. Commun. 15, 7835 (2024).
Hashibe, M. et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev.18, 541–550 (2009).
Pisani, P. et al. Metastatic disease in head & neck oncology. Acta Otorhinolaryngol. Ital. 40, S1–S86 (2020).
Wise-Draper, T. M., Bahig, H., Tonneau, M., Karivedu, V. & Burtness, B. Current therapy for metastatic head and neck cancer: evidence, opportunities, and challenges. in American Society of Clinical Oncology Educational Book 527–540. https://doi.org/10.1200/edbk_350442 (2022).
Pulte, D. & Brenner, H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist 15, 994–1001 (2010).
León, X. et al. Trends in disease-specific survival of head and neck squamous cell carcinoma patients treated in a single institution over a 30-year period. Oral Oncol. 115, 105184 (2021).
Sun, L. V. et al. Overall survival, treatment duration, and rechallenge outcomes with ICI therapy for recurrent or metastatic HNSCC. JAMA Netw. Open 7, e2428526 (2024).
Harrington, K. J. et al. Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: updated results of the phase III KEYNOTE-048 study. J. Clin. Oncol. 41, 790–802 (2023).
Elmusrati, A., Wang, J. & Wang, C. Y. Tumor microenvironment and immune evasion in head and neck squamous cell carcinoma. Int. J. Oral Sci. 13, 24 (2021).
Ruffin, A. T. et al. Improving head and neck cancer therapies by immunomodulation of the tumour microenvironment. Nat. Rev. Cancer 23, 173–188 (2023).
Ferris, R. L. et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 375, 1856–1867 (2016).
Seiwert, T. Y. et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 17, 956–965 (2016).
Burtness, B. et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394, 1915–1928 (2019).
Doroshow, D. B. et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 18, 345–362 (2021).
Burtness, B. et al. Pembrolizumab alone or with chemotherapy for recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE-048: subgroup analysis by programmed death ligand-1 combined positive score. J. Clin. Oncol. 40, 2321 (2022).
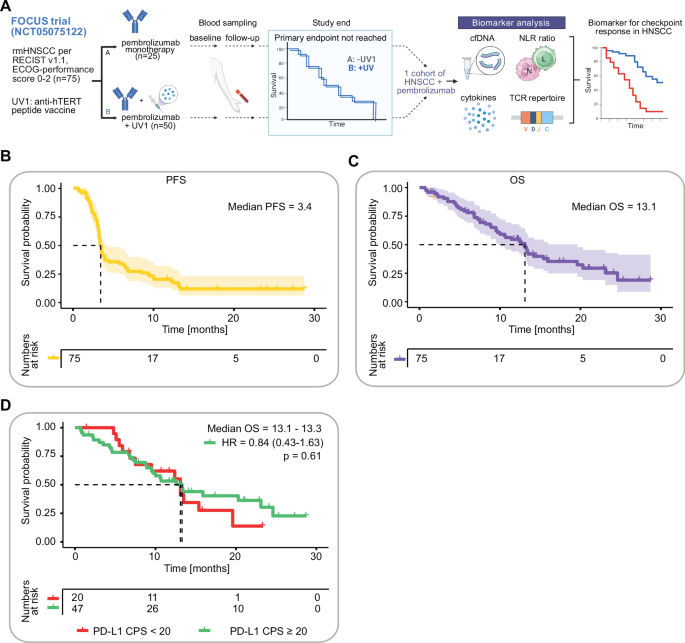
Brandt, A. et al. UV1 vaccination in pembrolizumab-treated patients with recurrent or metastatic head and neck cancer: a randomized multicenter phase 2 trial. Med 100647. https://doi.org/10.1016/j.medj.2025.100647 (2025).
Blazek, T., Petras, M., Knybel, L., Cvek, J. & Soumarova, R. Programmed cell death ligand 1 expression on immune cells and survival in patients with nonmetastatic head and neck cancer: a systematic review and meta-analysis. JAMA Netw. Open 6, e236324 (2023).
Paolino, G. et al. PD-L1 evaluation in head and neck squamous cell carcinoma: Insights regarding specimens, heterogeneity and therapy. Pathol. Res. Pract. 226, 153605 (2021).
Paschold, L. et al. First-line treatment of unresectable or metastatic HER2 positive esophagogastric adenocarcinoma: liquid biomarker analysis of the phase 2 INTEGA trial. J. Immunother. Cancer 11, https://doi.org/10.1136/jitc-2023-006678 (2023).
Akyuz, N. et al. T-cell diversification reflects antigen selection in the blood of patients on immune checkpoint inhibition and may be exploited as liquid biopsy biomarker. Int. J. Cancer 140, 2535–2544 (2017).
Simnica, D. et al. Responsiveness to immune checkpoint inhibitors is associated with a peripheral blood T-cell signature in metastatic castration-resistant prostate cancer. JCO Precis. Oncol. 4, 1374–1385 (2020).
Thuss-Patience, P. et al. Ramucirumab, avelumab, and paclitaxel as second-line treatment in esophagogastric adenocarcinoma: the phase 2 RAP (AIO-STO-0218) nonrandomized controlled trial. JAMA Netw. Open 7, e2352830 (2024).
Tintelnot, J. et al. Translational analysis and final efficacy of the AVETUX trial—avelumab, cetuximab and FOLFOX in metastatic colorectal cancer. Front. Oncol. 12, 993611 (2022).
Simnica, D. et al. T cell receptor next-generation sequencing reveals cancer-associated repertoire metrics and reconstitution after chemotherapy in patients with hematological and solid tumors. OncoImmunology 1–11. https://doi.org/10.1080/2162402X.2019.1644110 (2019).
Schultheiss, C. et al. Next-generation sequencing of T and B cell receptor repertoires from COVID-19 patients showed signatures associated with severity of disease. Immunity 53, 442–455 e444 (2020).
Simnica, D. et al. Landscape of T-cell repertoires with public COVID-19-associated T-cell receptors in pre-pandemic risk cohorts. Clin. Transl. Immunol. 10, e1340 (2021).
Bonomi, M., Patsias, A., Posner, M. & Sikora, A. The role of inflammation in head and neck cancer. Adv. Exp. Med. Biol. 816, 107–127 (2014).
Gu, D. Q., Ao, X., Yang, Y., Chen, Z. & Xu, X. Soluble immune checkpoints in cancer: production, function and biological significance. J. Immunother. Cancer 6, 132 (2018).
Chen, L., Chao, Y. Q., Li, W. J., Wu, Z. X. & Wang, Q. C. Soluble immune checkpoint molecules in cancer risk, outcomes prediction, and therapeutic applications. Biomark. Res. 12, 95 (2024).
Schultheiss, C. et al. Immune signatures in variant syndromes of primary biliary cholangitis and autoimmune hepatitis. Hepatol. Commun. 7, https://doi.org/10.1097/HC9.0000000000000123 (2023).
Mattox, A. K. et al. The origin of highly elevated cell-free DNA in healthy individuals and patients with pancreatic, colorectal, lung, or ovarian cancer. Cancer Discov. 13, 2166–2179 (2023).
Tintelnot, J. et al. Inflammatory stress determines the need for chemotherapy in patients with HER2-positive esophagogastric adenocarcinoma receiving targeted therapy and immunotherapy. Cancer Immunol. Res. 13, 200–209, https://doi.org/10.1158/2326-6066.CIR-24-0561 (2025).
Choudhary, M. M., France, T. J., Teknos, T. N. & Kumar, P. Interleukin-6 role in head and neck squamous cell carcinoma progression. World J. Otorhinolaryngol. Head. Neck Surg. 2, 90–97 (2016).
Huseni, M. A. et al. CD8(+) T cell-intrinsic IL-6 signaling promotes resistance to anti-PD-L1 immunotherapy. Cell Rep. Med. 4, 100878 (2023).
Hailemichael, Y. et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 40, 509–523 e506 (2022).
Soler, M. F., Abaurrea, A., Azcoaga, P., Araujo, A. M. & Caffarel, M. M. New perspectives in cancer immunotherapy: targeting IL-6 cytokine family. J. Immunother. Cancer 11, https://doi.org/10.1136/jitc-2023-007530 (2023).
Benhammadi, M. et al. IFN-lambda enhances constitutive expression of MHC class I molecules on thymic epithelial cells. J. Immunol. 205, 1268–1280 (2020).
Larsen, T. V., Daugaard, T. F., Gad, H. H., Hartmann, R. & Nielsen, A. L. PD-L1 and PD-L2 immune checkpoint protein induction by type III interferon in non-small cell lung cancer cells. Immunobiology 228, 152389 (2023).
Novotny, L. A. & Meissner, E. G. Expression and function of interferon lambda receptor 1 variants. FEBS Lett. 599, 466–475 (2025).
Lokau, J., Petasch, L. M. & Garbers, C. The soluble IL-2 receptor alpha/CD25 as a modulator of IL-2 function. Immunology 171, 377–387 (2024).
Chen, C. et al. Soluble Tim-3 serves as a tumor prognostic marker and therapeutic target for CD8(+) T cell exhaustion and anti-PD-1 resistance. Cell Rep. Med. 5, 101686 (2024).
Zak, J. et al. JAK inhibition enhances checkpoint blockade immunotherapy in patients with Hodgkin lymphoma. Science 384, eade8520 (2024).
Mathew, D. et al. Combined JAK inhibition and PD-1 immunotherapy for non-small cell lung cancer patients. Science 384, eadf1329 (2024).
Meyer, C. et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol. Immun. 63, 247–257 (2014).
Howard, R., Kanetsky, P. A. & Egan, K. M. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci. Rep. 9, 19673 (2019).
Sheng, I. Y. et al. Blood myeloid-derived suppressor cells correlate with neutrophil-to-lymphocyte ratio and overall survival in metastatic urothelial carcinoma. Target. Oncol. 15, 211–220 (2020).
Raskov, H., Orhan, A., Gaggar, S. & Gögenur, I. Neutrophils and polymorphonuclear myeloid-derived suppressor cells: an emerging battleground in cancer therapy. Oncogenesis 11, 22 (2022).
Templeton, A. J. et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J. Natl. Cancer Inst. 106, dju124 (2014).
Li, Q., Tie, Y., Alu, A., Ma, X. & Shi, H. Targeted therapy for head and neck cancer: signaling pathways and clinical studies. Signal Transduct. Target. Ther. 8, 31 (2023).
Bagchi, S., Yuan, R. & Engleman, E. G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. 16, 223–249 (2021).
Fortelny, N. et al. JAK-STAT signaling maintains homeostasis in T cells and macrophages. Nat. Immunol. 25, 847–859 (2024).
Sanz-Garcia, E., Zhao, E., Bratman, S. V. & Siu, L. L. Monitoring and adapting cancer treatment using circulating tumor DNA kinetics: Current research, opportunities, and challenges. Sci. Adv. 8, eabi8618 (2022).
Stanley, K. E. et al. Cell type signatures in cell-free DNA fragmentation profiles reveal disease biology. Nat. Commun. 15, 2220 (2024).
Rostami, A. et al. Senescence, necrosis, and apoptosis govern circulating cell-free DNA release kinetics. Cell Rep. 31, 107830 (2020).
Brandt, A. et al. Tolerability and efficacy of the cancer vaccine UV1 in patients with recurrent or metastatic PD-L1 positive head and neck squamous cell carcinoma planned for first-line treatment with pembrolizumab—the randomized phase 2 FOCUS trial. Front. Oncol. 14, 1283266 (2024).
Kassambara, A., Kosinski, M. & Biecek, P. survminer: Drawing Survival Curves using ‘ggplot2’. R package version 0.5.0.999, https://github.com/kassambara/survminer (2024).
Therneau, T. M. & Grambsch, P. M. Modeling Survival Data: Extending the Cox Model, https://therneau.r-universe.dev/survival (Springer New York, 2013).
Mährle, T. et al. Deep sequencing of bone marrow microenvironments of patients with del(5q) myelodysplastic syndrome reveals imprints of antigenic selection as well as generation of novel T cell clusters as a response pattern to lenalidomide. Haematologica https://doi.org/10.3324/haematol.2018.208223 (2019).
Mohme, M. et al. Immunophenotyping of newly diagnosed and recurrent glioblastoma defines distinct immune exhaustion profiles in peripheral and tumor-infiltrating lymphocytes. Clin. Cancer Res. 24, 4187–4200 (2018).
Schliffke, S. et al. Clinical response to ibrutinib is accompanied by normalization of the T-cell environment in CLL-related autoimmune cytopenia. Leukemia 30, 2232–2234 (2016).
Schliffke, S. et al. T-cell repertoire profiling by next-generation sequencing reveals tissue migration dynamics of TRBV13-family clonotypes in a common experimental autoimmune encephalomyelitis mouse model. J. Neuroimmunol. 332, 49–56 (2019).
Simnica, D. et al. High-throughput immunogenetics reveals a lack of physiological T cell clusters in patients with autoimmune cytopenias. Front. Immunol. 10, 1897 (2019).
Bolotin, D. A. et al. MiXCR: software for comprehensive adaptive immunity profiling. Nat. Methods 12, 380–381 (2015).
R Core Team. R: a language and environment for statistical computing. https://www.R-project.org (2018).
Nazarov, V. I. et al. tcR: an R package for T cell receptor repertoire advanced data analysis. BMC Bioinform. 16, 175 (2015).
Chiffelle, J. et al. T-cell repertoire analysis and metrics of diversity and clonality. Curr. Opin. Biotechnol. 65, 284–295 (2020).
Friedman, J. H., Hastie, T. & Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33, 1–22 (2010).
Blanche, P., Dartigues, J. F. & Jacqmin-Gadda, H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat. Med. 32, 5381–5397 (2013).