Product
Why Not Natural Pure Organic Moringa Green Superfood capsules with lot # A25G051 and expiration date 07/2028 marked on the bottom of the bottle have been recalled.
All Live it Up-brand Super Greens dietary supplement powder, including both original and wild berry flavors, with lots beginning with the letter “A” and all stick pack products with expiration dates from 08/2026 to 01/2028 have been recalled.
Additional products may be identified through FDA’s investigation. This advisory will be updated as more information becomes available.
Symptoms of Salmonella Infection
Illness usually occurs within 12 to 72 hours after eating food that is contaminated with Salmonella, and the symptoms usually last four to seven days. Symptoms include diarrhea, fever, and abdominal cramps. Children younger than five, the elderly, and people with weakened immune systems are more likely to have severe infections.
Stores affected
Recalled Why Not Natural Pure Organic Moringa Green Superfood capsules and Live it Up-brand Super Greens dietary supplement powder were primarily sold online on their company websites and other online sites including Amazon, eBay, and Walmart.
Status
Ongoing
RecommendationsConsumers and retailers should not eat, sell, or serve recalled Why Not Natural Pure Organic Moringa Green Superfood capsules (lot # A25G051 and expiration date 07/2028) or recalled Live it Up-brand Super Greens dietary supplement powder (original or wild berry flavor) with expiration dates from 08/2026 to 01/2028. Consumers should throw these products away and may request a refund by contacting the respective company.Consumers and retailers who purchased or received recalled dietary supplements should carefully clean and sanitize any surfaces or containers that the products touched. Follow FDA’s safe handling and cleaning advice and use extra care in cleaning and sanitizing any surfaces and containers that may have come in contact with these products to reduce the risk of cross-contamination. Contact your healthcare provider if you think you may have developed symptoms of a Salmonella infection after consuming recalled dietary supplements.
Current Update
January 29, 2026
The FDA and CDC, in collaboration with state and local partners, are investigating illnesses in a multistate outbreak of Salmonella Typhimurium and Salmonella Newport infections linked to recalled Why Not Natural Pure Organic Moringa Green Superfood capsules and recalled Live it Up-brand Super Greens dietary supplement powder.
Since the last update on January 15, 2026, a total of 20 new illnesses have been reported, including a new outbreak strain of Salmonella Newport. As of January 29, 2026, CDC reported a total of 65 people from 28 states that have been infected with one of the outbreak strains of Salmonella. Of the 40 people interviewed, 35 (88%) reported eating a product containing moringa leaf powder, including 31 who reported Live it Up Super Greens supplement powders only, 3 who reported Why Not Natural moringa powder capsules only, and 1 person who reported consuming both products. There have been 14 hospitalizations, and no deaths have been reported.
FDA’s traceback investigation revealed a common manufacturer between Live it Up-brand Super Greens and Why Not Natural Pure Organic Moringa Green Superfood capsules that used moringa leaf powder in both products. Epidemiologic and traceback evidence showed that moringa leaf powder is the source of contamination in this outbreak, and lot # A25G051 of Why Not Natural Pure Organic Moringa Green Superfood capsules with expiration date 07/2028 may be contaminated with the strains of Salmonella making people sick in this outbreak. On January 24, 2026, Art Monkey LLC dba Why Not Natural of Houston, TX stopped the sale of Pure Organic Moringa Green Superfood capsules and initiated a recall of lot # A25G051 on January 28, 2026. FDA is working with the firms to determine a root cause of the contamination and remove affected products from the market.
On January 20, 2026, Superfoods, Inc. expanded their recall of Live it Up-brand Super Greens product to include nationwide distribution of recalled product, including Puerto Rico, Guam, and the U.S. Virgin Islands, as well as international distribution to consumers in the United Kingdom. On January 26, 2026, Superfoods, Inc.’s recall press was updated to make changes to the net weight of recalled Live it Up-brand Super Greens Wild Berry products. See the recall notice for more information.
FDA’s investigation is ongoing. This advisory will be updated as more information becomes available.
Case Count Map Provided by CDC 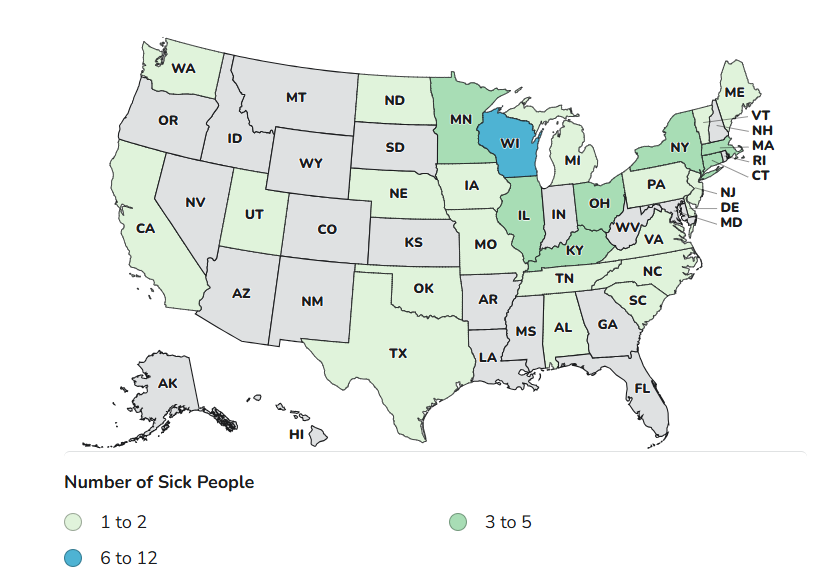
Case Counts
Total Illnesses: 65
Hospitalizations: 14
Deaths: 0
Last Illness Onset: January 11, 2026
States with Cases: AL, CA, CT, DE, IA, IL, KY, MA, ME, MI, MN, MO, NC, ND, NE, NJ, NY, OH, OK, PA, SC, TN, TX, UT, VA, VT, WA, WI
Product Distribution: Nationwide, including Puerto Rico, Guam, and the Virgin Islands



International Distribution
The Live it Up Super Greens recall impacts markets outside the United States. Customer information provided by the firm shows that recalled product was sold to consumers in the United Kingdom. The recalled product was also sold to consumers located nationwide in the United States, including Puerto Rico, Guam, and Virgin Islands.
Previous Updates
January 15, 2026
The FDA and CDC, in collaboration with state and local partners, are investigating illnesses in a multistate outbreak of Salmonella Typhimurium infections linked to Live it Up-brand Super Greens dietary supplement powder produced by Superfoods, Inc. doing business as (dba) Live it Up of New York, NY.
On January 15, 2026, Superfoods, Inc. recalled all Live it Up-brand Super Greens dietary supplement powder with expiration dates from 08/2026 to 01/2028.
The recall includes the following products:
Live it Up Super Greens, NET WT 8.5 oz (240g) with UPC 860013190804.Live it Up Super Greens, 30 – 0.28oz (8g) sticks, NET WT. 8.47 oz (240g) with UPC 850077468063Live it Up Super Greens, Wild Berry, NET WT 8.5OZ (240g), with UPC 860013190811Live it Up Super Greens, Wild Berry, 30 – 0.32oz (9g) Sticks, NET WT. 9.52oz (270g), with UPC 850077468070
Consumers and retailers who purchased recalled Live it Up-brand Super Greens products should not eat, sell, or serve this product and should throw it away. FDA is determining if additional recalls are necessary and will update this advisory as more information becomes available.
In addition, FDA is conducting a traceback investigation of products ill people reported consuming before becoming ill and is working with state partners to sample products of concern. FDA’s investigation is ongoing.
January 14, 2026
The FDA and CDC, in collaboration with state and local partners, are investigating illnesses in a multistate outbreak of Salmonella Typhimurium infections linked to Live it Up-brand Super Greens dietary supplement powder produced by Superfoods, Inc. doing business as (dba) Live it Up of New York, NY.
Based on epidemiological information collected by CDC, a total of 45 people infected with the outbreak strain of Salmonella have been reported from 21 states. Illnesses started on dates ranging from August 22, 2025, to December 30, 2025. Sixteen of 20 ill persons with information available reported consuming Live it Up-brand Super Greens dietary supplement powder before becoming ill. There have been 12 hospitalizations, and no deaths have been reported.
FDA has recommended that Superfoods, Inc. dba Live it Up recall Live it Up-brand Super Greens dietary supplement powder (original and wild berry flavor) products from the market. On January 14, 2026, the firm agreed to initiate a voluntary recall. Consumers and retailers who purchased Live it Up-brand Super Greens dietary supplement powder with expiration dates from 08/2026 to 01/2028 should not eat, sell, or serve this product and should throw it away or return it to the place of purchase.
To determine a source of contamination, FDA is conducting a traceback investigation of products ill people reported consuming before becoming ill and is working with state partners to sample products of concern. Additional products may be contaminated, and this advisory will be updated as more information becomes available.
Consumers who have symptoms should contact their health care provider to report their symptoms and receive care.
To report a complaint or adverse event (illness or serious allergic reaction),
visit Industry and Consumer Assistance.
Follow us on X (formerly Twitter)