Crucian, B. et al. Alterations in adaptive immunity persist during long-duration spaceflight. npj Microgravity 1, 15013 (2015).
Konstantinova, I. V., Rykova, M. P., Lesnyak, A. T. & Antropova, E. A. Immune changes during long-duration missions. J. Leukoc. Biol. 54, 189–201 (1993).
Mehta, S. K. et al. Multiple latent viruses reactivate in astronauts during space shuttle missions. Brain Behav. Immun. 41, 210–217 (2014).
Mehta, S. K. et al. Latent virus reactivation in astronauts on the International Space Station. npj Microgravity 3, 11 (2017).
Nickerson, C. A., Ott, C. M., Wilson, J. W., Ramamurthy, R. & Pierson, D. L. Microbial responses to microgravity and other low-shear environments. Microbiol. Mol. Biol. Rev. 68, 345–361 (2004).
Mason, C. E. et al. A second space age spanning omics, platforms and medicine across orbits. Nature 632, 995–1008 (2024). The ‘second space age’ is driving the integration of cutting-edge molecular biology and precision medicine into aerospace health care, fundamentally transforming astronaut well-being for extended missions, especially through comprehensive data collection and biobanking efforts such as the SOMA.
Rooney, B. V., Crucian, B. E., Pierson, D. L., Laudenslager, M. L. & Mehta, S. K. Herpes virus reactivation in astronauts during spaceflight and its application on earth. Front. Microbiol. 10, 16 (2019).
Crucian, B. et al. Immune system dysregulation occurs during short duration spaceflight on board the space shuttle. J. Clin. Immunol. 33, 456–465 (2013).
Crucian, B. et al. Incidence of clinical symptoms during long-duration orbital spaceflight. Int. J. Gen. Med. 9, 383–391 (2016).
Penley, N. J., Schafer, C. P. & Bartoe, J.-D. F. The International Space Station as a microgravity research platform. Acta Astronaut. 50, 691–696 (2002).
Lv, H. et al. Microgravity and immune cells. J. R. Soc. Interface 20, 20220869 (2023).
Bonanni, R., Cariati, I., Marini, M., Tarantino, U. & Tancredi, V. Microgravity and musculoskeletal health: what strategies should be used for a great challenge? Life 13, 1423 (2023).
Nelson, E. S., Mulugeta, L. & Myers, J. G. Microgravity-induced fluid shift and ophthalmic changes. Life 4, 621–665 (2014).
Martinez, E. M., Yoshida, M. C., Candelario, T. L. T. & Hughes-Fulford, M. Spaceflight and simulated microgravity cause a significant reduction of key gene expression in early T-cell activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R480–R488 (2015). An early study comparing the effects of simulated microgravity with true microgravity in spaceflight on T cell gene expression post-activation, demonstrating reduced T cell activation by microgravity.
Chang, T. T., Spurlock, S. M., Candelario, T. L. T., Grenon, S. M. & Hughes-Fulford, M. Spaceflight impairs antigen-specific tolerance induction in vivo and increases inflammatory cytokines. FASEB J. 29, 4122–4132 (2015).
Hicks, J., Olson, M., Mitchell, C., Juran, C. M. & Paul, A. M. The impact of microgravity on immunological states. Immunohorizons 7, 670–682 (2023).
Li, Q. et al. Effects of simulated microgravity on primary human NK cells. Astrobiology 13, 703–714 (2013).
Bigley, A. B. et al. NK cell function is impaired during long-duration spaceflight. J. Appl. Physiol. 126, 842–853 (2019).
Kuhlman, B. M. et al. Simulated microgravity impairs human NK cell cytotoxic activity against space radiation-relevant leukemic cells. npj Microgravity 10, 85 (2024).
Thiel, C. S. et al. Rapid adaptation to microgravity in mammalian macrophage cells. Sci. Rep. 7, 43 (2017).
Shi, L. et al. Spaceflight and simulated microgravity suppresses macrophage development via altered RAS/ERK/NFκB and metabolic pathways. Cell. Mol. Immunol. 18, 1489–1502 (2021).
Savary, C. A. et al. Characteristics of human dendritic cells generated in a microgravity analog culture system. In Vitr. Cell Dev. Biol. Anim. 37, 216–222 (2001).
Kaur, I., Simons, E. R., Castro, V. A., Mark Ott, C. & Pierson, D. L. Changes in neutrophil functions in astronauts. Brain Behav. Immun. 18, 443–450 (2004).
Paul, A. M. et al. Neutrophil-to-lymphocyte ratio: a biomarker to monitor the immune status of astronauts. Front. Immunol. 11, 564950 (2020).
Jacob, P., Bonnefoy, J., Ghislin, S. & Frippiat, J.-P. Long-duration head-down tilt bed rest confirms the relevance of the neutrophil to lymphocyte ratio and suggests coupling it with the platelet to lymphocyte ratio to monitor the immune health of astronauts. Front. Immunol. 13, 952928 (2022).
Dai, S. et al. Effect of simulated microgravity conditions of hindlimb unloading on mice hematopoietic and mesenchymal stromal cells. Cell Biol. Int. 44, 2243–2252 (2020).
Ezura, Y. et al. Hindlimb-unloading suppresses B cell population in the bone marrow and peripheral circulation associated with OPN expression in circulating blood cells. J. Bone Miner. Metab. 33, 48–54 (2015).
Zhou, Y. et al. Effect of solar particle event radiation and hindlimb suspension on gastrointestinal tract bacterial translocation and immune activation. PLoS ONE 7, e44329 (2012).
Shi, J. et al. Intestinal microbiota contributes to colonic epithelial changes in simulated microgravity mouse model. FASEB J. 31, 3695–3709 (2017).
Davis, T. A. et al. Effect of spaceflight on human stem cell hematopoiesis: suppression of erythropoiesis and myelopoiesis. J. Leukoc. Biol. 60, 69–76 (1996).
Trudel, G., Melkus, G. & Liu, T. The ups and downs of bone-marrow adipose tissue in space. Trends Endocrinol. Metab. 35, 85–87 (2024).
Akiyama, T. et al. How does spaceflight affect the acquired immune system? npj Microgravity 6, 14 (2020).
Woods, C. C., Banks, K. E., Gruener, R. & DeLuca, D. Loss of T cell precursors after spaceflight and exposure to vector-averaged gravity. FASEB J. 17, 1526–1528 (2003).
Muramatsu, W., Maryanovich, M., Akiyama, T. & Karagiannis, G. S. Thymus ad astra, or spaceflight-induced thymic involution. Front. Immunol. 15, 1534444 (2024).
Luo, H., Wang, C., Feng, M. & Zhao, Y. Microgravity inhibits resting T cell immunity in an exposure time-dependent manner. Int. J. Med. Sci. 11, 87–96 (2014).
Hashemi, B. B. et al. T cell activation responses are differentially regulated during clinorotation and in spaceflight. FASEB J. 13, 2071–2082 (1999).
Tauber, S. et al. Signal transduction in primary human T lymphocytes in altered gravity — results of the MASER-12 suborbital space flight mission. Cell Commun. Signal. 11, 32 (2013).
Tauber, S. et al. Signal transduction in primary human T lymphocytes in altered gravity during parabolic flight and clinostat experiments. Cell. Physiol. Biochem. 35, 1034–1051 (2015).
Spatz, J. M. et al. Human immune system adaptations to simulated microgravity revealed by single-cell mass cytometry. Sci. Rep. 11, 11872 (2021).
Spielmann, G. et al. B cell homeostasis is maintained during long-duration spaceflight. J. Appl. Physiol. 126, 469–476 (2019).
Gaignier, F. et al. Three weeks of murine hindlimb unloading induces shifts from B to T and from Th to Tc splenic lymphocytes in absence of stress and differentially reduces cell-specific mitogenic responses. PLoS ONE 9, e92664 (2014).
Wilson, J. M. et al. Comparison of hindlimb unloading and partial weight suspension models for spaceflight-type condition induced effects on white blood cells. Adv. Space Res. 49, 237–248 (2012).
Wei, L. X., Zhou, J. N., Roberts, A. I. & Shi, Y. F. Lymphocyte reduction induced by hindlimb unloading: distinct mechanisms in the spleen and thymus. Cell Res. 13, 465–471 (2003).
Cao, D. et al. Hematopoietic stem cells and lineage cells undergo dynamic alterations under microgravity and recovery conditions. FASEB J. 33, 6904–6918 (2019).
Lescale, C. et al. Hind limb unloading, a model of spaceflight conditions, leads to decreased B lymphopoiesis similar to aging. FASEB J. 29, 455–463 (2015).
Wu, F. et al. Single-cell analysis identifies conserved features of immune dysfunction in simulated microgravity and spaceflight. Nat. Commun. 15, 4795 (2024). The first single-cell analysis of the human immune system from simulated microgravity with validation to spaceflight data, including the SpaceX Inspiration4 mission, NASA Twins and JAXA cell-free epigenome study, identifies core hallmarks of immune dysfunction in microgravity and spaceflight. This paper also used machine learning to identify nutritional countermeasures to reverse the effects of microgravity on immune cells.
da Silveira, W. A. et al. Comprehensive multi-omics analysis reveals mitochondrial stress as a central biological hub for spaceflight impact. Cell 183, 1185–1201.e20 (2020). The first large multiomics assessment across multiple astronauts, different tissue types and multiple modelled systems identifies key alterations in organism biology, including mitochondrial dysfunction, induced by spaceflight.
Murali, A. & Sarkar, R. R. Mechano-immunology in microgravity. Life Sci. Space Res. 37, 50–64 (2023).
Du, H. et al. Tuning immunity through tissue mechanotransduction. Nat. Rev. Immunol. 23, 174–188 (2023).
Neelam, S. et al. Changes in nuclear shape and gene expression in response to simulated microgravity are LINC complex-dependent. Int. J. Mol. Sci. 21, 6762 (2020).
Thiel, C. S. et al. Rapid coupling between gravitational forces and the transcriptome in human myelomonocytic U937 cells. Sci. Rep. 8, 13267 (2018).
Vahlensieck, C., Thiel, C. S., Zhang, Y., Huge, A. & Ullrich, O. Gravitational force-induced 3D chromosomal conformational changes are associated with rapid transcriptional response in human T cells. Int. J. Mol. Sci. 22, 9426 (2021).
Sciola, L., Cogoli-Greuter, M., Cogoli, A., Spano, A. & Pippia, P. Influence of microgravity on mitogen binding and cytoskeleton in Jurkat cells. Adv. Space Res. 24, 801–805 (1999).
Papaseit, C., Pochon, N. & Tabony, J. Microtubule self-organization is gravity-dependent. Proc. Natl Acad. Sci. USA 97, 8364–8368 (2000).
Schatten, H., Lewis, M. L. & Chakrabarti, A. Spaceflight and clinorotation cause cytoskeleton and mitochondria changes and increases in apoptosis in cultured cells. Acta Astronaut. 49, 399–418 (2001).
Trono, P., Tocci, A., Musella, M., Sistigu, A. & Nisticò, P. Actin cytoskeleton dynamics and type I IFN-mediated immune response: a dangerous liaison in cancer? Biology 10, 913 (2021).
El Masri, R. & Delon, J. RHO GTPases: from new partners to complex immune syndromes. Nat. Rev. Immunol. 21, 499–513 (2021).
Liu, S. et al. RhoB induces the production of proinflammatory cytokines in TLR-triggered macrophages. Mol. Immunol. 87, 200–206 (2017).
Ohman, T., Rintahaka, J., Kalkkinen, N., Matikainen, S. & Nyman, T. A. Actin and RIG-I/MAVS signaling components translocate to mitochondria upon influenza a virus infection of human primary macrophages. J. Immunol. 182, 5682–5692 (2009).
Mukherjee, A. et al. Retinoic acid-induced gene-1 (RIG-I) associates with the actin cytoskeleton via caspase activation and recruitment domain-dependent interactions. J. Biol. Chem. 284, 6486–6494 (2009).
Zhu, L. et al. Attenuation of antiviral immune response caused by perturbation of TRIM25-mediated RIG-I activation under simulated microgravity. Cell Rep. 34, 108600 (2021).
Guignandon, A. et al. Rac1 GTPase silencing counteracts microgravity-induced effects on osteoblastic cells. FASEB J. 28, 4077–4087 (2014).
Husna, N. et al. Release of CD36-associated cell-free mitochondrial DNA and RNA as a hallmark of space environment response. Nat. Commun. 15, 4814 (2024).
Hatton, J. P., Gaubert, F., Cazenave, J.-P. & Schmitt, D. Microgravity modifies protein kinase C isoform translocation in the human monocytic cell line U937 and human peripheral blood T-cells. J. Cell. Biochem. 87, 39–50 (2002).
Larsson, C. Protein kinase C and the regulation of the actin cytoskeleton. Cell. Signal. 18, 276–284 (2006).
Hui, X., Sauer, B., Kaestner, L., Kruse, K. & Lipp, P. PKCα diffusion and translocation are independent of an intact cytoskeleton. Sci. Rep. 7, 475 (2017).
Garrett-Bakelman, F. E. et al. The NASA Twins study: a multidimensional analysis of a year-long human spaceflight. Science 364, eaau8650 (2019). Analysing a 340-day space mission on a twin astronaut, the NASA Twins Study revealed extensive integrated, longitudinal physiological and molecular changes of the effects of spaceflight on a human subject.
Van den Bossche, J., O’Neill, L. A. & Menon, D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 38, 395–406 (2017).
Zhao, T. V., Sato, Y., Goronzy, J. J. & Weyand, C. M. T-cell aging-associated phenotypes in autoimmune disease. Front. Aging 3, 867950 (2022).
Locatelli, L., Cazzaniga, A., De Palma, C., Castiglioni, S. & Maier, J. A. M. Mitophagy contributes to endothelial adaptation to simulated microgravity. FASEB J. 34, 1833–1845 (2020).
Nguyen, H. P., Tran, P. H., Kim, K.-S. & Yang, S.-G. The effects of real and simulated microgravity on cellular mitochondrial function. npj Microgravity 7, 44 (2021).
Tauber, S. et al. Cytoskeletal stability and metabolic alterations in primary human macrophages in long-term microgravity. PLoS ONE 12, e0175599 (2017).
Prado, L. G., Camara, N. O. S. & Barbosa, A. S. Cell lipid biology in infections: an overview. Front. Cell. Infect. Microbiol. 13, 1148383 (2023).
Jain, N. & Vogel, V. Spatial confinement downsizes the inflammatory response of macrophages. Nat. Mater. 17, 1134–1144 (2018).
Kim, J. et al. Single-cell multi-ome and immune profiles of the Inspiration4 crew reveal conserved, cell-type, and sex-specific responses to spaceflight. Nat. Commun. 15, 4954 (2024). This study categorized the immune response changes of four SpaceX Inspiration4 mission crewmembers using single-cell multiomics analysis, revealing spaceflight-induced alterations in gene expression, chromatin accessibility and immune cell proportions. It further identified a conserved spaceflight signature showing dysregulation in oxidative phosphorylation, translation, UV response and TCF21 pathways.
Wernlé, K., Thiel, C. S. & Ullrich, O. Increased H3K9me3 and F-actin reorganization in the rapid adaptive response to hypergravity in human T lymphocytes. Int. J. Mol. Sci. 24, 17232 (2023).
Vahlensieck, C. et al. Post-transcriptional dynamics is involved in rapid adaptation to hypergravity in jurkat T cells. Front. Cell Dev. Biol. 10, 933984 (2022).
Lang, A. et al. Acute and short-term fluctuations in gravity are associated with changes in circulatory plasma protein levels. npj Microgravity 10, 25 (2024).
Ghislin, S., Ouzren-Zarhloul, N., Kaminski, S. & Frippiat, J.-P. Hypergravity exposure during gestation modifies the TCRβ repertoire of newborn mice. Sci. Rep. 5, 9318 (2015).
Guéguinou, N. et al. Stress response and humoral immune system alterations related to chronic hypergravity in mice. Psychoneuroendocrinology 37, 137–147 (2012).
Moser, D. et al. Differential effects of hypergravity on immune dysfunctions induced by simulated microgravity. FASEB J. 37, e22910 (2023).
McDonald, J. T. et al. NASA GeneLab platform utilized for biological response to space radiation in animal models. Cancers 12, 381 (2020).
Paul, A. M. et al. Beyond low-Earth orbit: characterizing immune and microRNA differentials following simulated deep spaceflight conditions in mice. iScience 23, 101747 (2020).
Burke, M. et al. Sexual dimorphism during integrative endocrine and immune responses to ionizing radiation in mice. Sci. Rep. 14, 7334 (2024).
Rienecker, K. D. A. et al. Combined space stressors induce independent behavioral deficits predicted by early peripheral blood monocytes. Sci. Rep. 13, 1749 (2023).
Sanzari, J. K., Cengel, K. A., Wan, X. S., Rusek, A. & Kennedy, A. R. Acute hematological effects in mice exposed to the expected doses, dose-rates, and energies of solar particle event-like proton radiation. Life Sci. Space Res. 2, 86–91 (2014).
Gridley, D. S., Obenaus, A., Bateman, T. A. & Pecaut, M. J. Long-term changes in rat hematopoietic and other physiological systems after high-energy iron ion irradiation. Int. J. Radiat. Biol. 84, 549–559 (2008).
Gridley, D. S., Pecaut, M. J., Dutta-Roy, R. & Nelson, G. A. Dose and dose rate effects of whole-body proton irradiation on leukocyte populations and lymphoid organs: part I. Immunol. Lett. 80, 55–66 (2002).
Gridley, D. S., Pecaut, M. J. & Nelson, G. A. Total-body irradiation with high-LET particles: acute and chronic effects on the immune system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R677–R688 (2002).
Pecaut, M. J. et al. Acute effects of iron-particle radiation on immunity. Part I: population distributions. Radiat. Res. 165, 68–77 (2006).
Gridley, D. S. et al. Low-dose photon and simulated solar particle event proton effects on Foxp3+ T regulatory cells and other leukocytes. Technol. Cancer Res. Treat. 9, 637–649 (2010).
Dang, B. et al. Simulated microgravity increases heavy ion radiation-induced apoptosis in human B lymphoblasts. Life Sci. 97, 123–128 (2014).
Gridley, D. S. et al. Spaceflight effects on T lymphocyte distribution, function and gene expression. J. Appl. Physiol. 106, 194–202 (2009).
Crucian, B. E. et al. Immune system dysregulation during spaceflight: potential countermeasures for deep space exploration missions. Front. Immunol. 9, 1437 (2018). Human immune dysfunction and latent herpesvirus reactivation are frequently observed during orbital spaceflight. This review discusses existing and potential countermeasures, including nutritional, pharmacological and vaccination strategies, to mitigate clinical risks and leverage precision medicine for protecting astronaut health during future long-duration and deep space exploration missions.
Nzabarushimana, E. et al. Combined exposure to protons and 56Fe leads to overexpression of Il13 and reactivation of repetitive elements in the mouse lung. Life Sci. Space Res. 7, 1–8 (2015).
Rodman, C. et al. In vitro and in vivo assessment of direct effects of simulated solar and galactic cosmic radiation on human hematopoietic stem/progenitor cells. Leukemia 31, 1398–1407 (2017).
Moreno-Villanueva, M., Wong, M., Lu, T., Zhang, Y. & Wu, H. Interplay of space radiation and microgravity in DNA damage and DNA damage response. npj Microgravity 3, 14 (2017).
Beheshti, A. et al. Genomic changes driven by radiation-induced DNA damage and microgravity in human cells. Int. J. Mol. Sci. 22, 10507 (2021).
Pariset, E. et al. DNA damage baseline predicts resilience to space radiation and radiotherapy. Cell Rep. 33, 108434 (2020).
Feiveson, A. et al. Predicting chromosome damage in astronauts participating in International Space Station missions. Sci. Rep. 11, 5293 (2021).
Heylmann, D., Ponath, V., Kindler, T. & Kaina, B. Comparison of DNA repair and radiosensitivity of different blood cell populations. Sci. Rep. 11, 2478 (2021).
Limoli, C. L., Ponnaiya, B., Corcoran, J. J., Giedzinski, E. & Morgan, W. F. Chromosomal instability induced by heavy ion irradiation. Int. J. Radiat. Biol. 76, 1599–1606 (2000).
Ding, L.-H. et al. Distinct transcriptome profiles identified in normal human bronchial epithelial cells after exposure to γ-rays and different elemental particles of high Z and energy. BMC Genomics 14, 372 (2013).
Kidane, D. et al. Interplay between DNA repair and inflammation, and the link to cancer. Crit. Rev. Biochem. Mol. Biol. 49, 116–139 (2014).
Cucinotta, F. A. & Cacao, E. Non-targeted effects models predict significantly higher Mars mission cancer risk than targeted effects models. Sci. Rep. 7, 1832 (2017).
Ikeda, H. et al. Expression profile of cell cycle-related genes in human fibroblasts exposed simultaneously to radiation and simulated microgravity. Int. J. Mol. Sci. 20, 4791 (2019).
Stowe, R. P., Sams, C. F. & Pierson, D. L. Effects of mission duration on neuroimmune responses in astronauts. Aviat. Space Environ. Med. 74, 1281–1284 (2003).
Reale, M. et al. Network between cytokines, cortisol and occupational stress in gas and oilfield workers. Int. J. Mol. Sci. 21, 1118 (2020).
Kaur, I., Simons, E. R., Kapadia, A. S., Ott, C. M. & Pierson, D. L. Effect of spaceflight on ability of monocytes to respond to endotoxins of Gram-negative bacteria. Clin. Vaccin. Immunol. 15, 1523–1528 (2008).
Crucian, B. E. et al. Plasma cytokine concentrations indicate that in vivo hormonal regulation of immunity is altered during long-duration spaceflight. J. Interferon Cytokine Res. 34, 778–786 (2014).
Benjamin, C. L. et al. Decreases in thymopoiesis of astronauts returning from space flight. JCI Insight 1, e88787 (2016).
Eckberg, D. L. & Neurolab Autonomic Nervous System Team. Bursting into space: alterations of sympathetic control by space travel. Acta Physiol. Scand. 177, 299–311 (2003).
Carnagarin, R., Matthews, V., Zaldivia, M. T. K., Peter, K. & Schlaich, M. P. The bidirectional interaction between the sympathetic nervous system and immune mechanisms in the pathogenesis of hypertension. Br. J. Pharmacol. 176, 1839–1852 (2019).
Bellocchi, C. et al. The interplay between autonomic nervous system and inflammation across systemic autoimmune diseases. Int. J. Mol. Sci. 23, 2449 (2022).
Miranda, S. et al. Lost in space? Unmasking the T cell reaction to simulated space stressors. Int. J. Mol. Sci. 24, 16943 (2023).
Malatesta, P. et al. Differential gene expression in human fibroblasts simultaneously exposed to ionizing radiation and simulated microgravity. Biomolecules 14, 88 (2024).
Davidson, S. et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat. Rev. Immunol. 21, 704–717 (2021).
Wadhwa, A., Moreno-Villanueva, M., Crucian, B. & Wu, H. Synergistic interplay between radiation and microgravity in spaceflight-related immunological health risks. Immun. Ageing 21, 50 (2024).
Malkani, S. et al. Circulating miRNA spaceflight signature reveals targets for countermeasure development. Cell Rep. 33, 108448 (2020).
Nickerson, C. A. et al. Microbiology of human spaceflight: microbial responses to mechanical forces that impact health and habitat sustainability. Microbiol. Mol. Biol. Rev. 88, e0014423 (2024).
Guo, H. et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 370, eaay9097 (2020).
Moraitis, I., Guiu, J. & Rubert, J. Gut microbiota controlling radiation-induced enteritis and intestinal regeneration. Trends Endocrinol. Metab. 34, 489–501 (2023).
Hills, R. D. et al. Gut microbiome: profound implications for diet and disease. Nutrients 11, 1613 (2019).
Yang, J. et al. Navigating mental health in space: gut–brain axis and microbiome dynamics. Exp. Mol. Med. 57, 1152–1163 (2025).
Morrison, M. D. et al. Investigation of spaceflight induced changes to astronaut microbiomes. Front. Microbiol. 12, 659179 (2021). This study used shotgun metagenomic sequencing and microarrays on multisite body samples from four ISS astronauts to reveal individual-specific microbiome shifts and a notable post-flight increase in antimicrobial resistance genes, demonstrating these technologies’ utility for space microbiome monitoring.
Voorhies, A. A. et al. Study of the impact of long-duration space missions at the International Space Station on the astronaut microbiome. Sci. Rep. 9, 9911 (2019).
Malli Mohan, G. B. et al. Microbiome and metagenome analyses of a closed habitat during human occupation. mSystems 5, e00367–20 (2020).
Checinska Sielaff, A. et al. Characterization of the total and viable bacterial and fungal communities associated with the International Space Station surfaces. Microbiome 7, 50 (2019).
Salido, R. A. et al. The International Space Station has a unique and extreme microbial and chemical environment driven by use patterns. Cell 188, 2022–2041.e23 (2025). A comprehensive mapping of microbial composition on numerous surfaces of the US Orbital Segment of the ISS identifies key microbial changes linked to ISS modulate function and the effects of chemicals, with implications to immunity.
Avila-Herrera, A. et al. Crewmember microbiome may influence microbial composition of ISS habitable surfaces. PLoS ONE 15, e0231838 (2020).
Tierney, B. T. et al. Longitudinal multi-omics analysis of host microbiome architecture and immune responses during short-term spaceflight. Nat. Microbiol. 9, 1661–1675 (2024). Results from the Inspiration4 mission utilizing extensive mapping of microbial changes with relation to predicted effects on immunity across different body sites in response to short-duration spaceflight.
Liu, Z. et al. Effects of spaceflight on the composition and function of the human gut microbiota. Gut Microbes 11, 807–819 (2020).
Jiang, P., Green, S. J., Chlipala, G. E., Turek, F. W. & Vitaterna, M. H. Reproducible changes in the gut microbiome suggest a shift in microbial and host metabolism during spaceflight. Microbiome 7, 113 (2019).
Trudel, G., Shahin, N., Ramsay, T., Laneuville, O. & Louati, H. Hemolysis contributes to anemia during long-duration space flight. Nat. Med. 28, 59–62 (2022).
Mehta, S. K., Stowe, R. P., Feiveson, A. H., Tyring, S. K. & Pierson, D. L. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J. Infect. Dis. 182, 1761–1764 (2000). This study reported early evidence that cytomegalovirus reactivation and shedding occurred in 71 astronauts both before and during spaceflight, shown by significantly increased shedding frequency in urine and increased antibody titres.
Mehta, S. K. et al. Reactivation of latent viruses is associated with increased plasma cytokines in astronauts. Cytokine 61, 205–209 (2013).
Mehta, S. K. et al. Dermatitis during spaceflight associated with HSV-1 reactivation. Viruses 14, 789 (2022).
Pierson, D. L., Stowe, R. P., Phillips, T. M., Lugg, D. J. & Mehta, S. K. Epstein–Barr virus shedding by astronauts during space flight. Brain Behav. Immun. 19, 235–242 (2005).
Mehta, S. K. & Pierson, D. L. Reactivation of latent herpes viruses in cosmonauts during a Soyuz taxi mission. Microgravity Sci. Technol. 19, 215–218 (2007).
Crucian, B. E. et al. Countermeasures-based improvements in stress, immune system dysregulation and latent herpesvirus reactivation onboard the International Space Station — relevance for deep space missions and terrestrial medicine. Neurosci. Biobehav. Rev. 115, 68–76 (2020).
Spielmann, G. et al. Latent viral reactivation is associated with changes in plasma antimicrobial protein concentrations during long-duration spaceflight. Acta Astronaut. 146, 111–116 (2018).
Kunz, H. E. et al. Zoster patients on earth and astronauts in space share similar immunologic profiles. Life Sci. Space Res. 25, 119–128 (2020).
Urbaniak, C. et al. The influence of spaceflight on the astronaut salivary microbiome and the search for a microbiome biomarker for viral reactivation. Microbiome 8, 56 (2020).
Tibbetts, S. A., van Dyk, L. F., Speck, S. H. & Virgin, H. W. Immune control of the number and reactivation phenotype of cells latently infected with a gammaherpesvirus. J. Virol. 76, 7125–7132 (2002).
Gasser, O. et al. Treatment-dependent loss of polyfunctional CD8+ T-cell responses in HIV-infected kidney transplant recipients is associated with herpesvirus reactivation. Am. J. Transplant. 9, 794–803 (2009).
Crucian, B. E., Stowe, R. P., Pierson, D. L. & Sams, C. F. Immune system dysregulation following short- vs long-duration spaceflight. Aviat. Space Environ. Med. 79, 835–843 (2008).
Mehta, S. K. et al. Reactivation of latent Epstein–Barr virus: a comparison after exposure to gamma, proton, carbon, and iron radiation. Int. J. Mol. Sci. 19, 2961 (2018).
Tascher, G. et al. Analysis of femurs from mice embarked on board BION-M1 biosatellite reveals a decrease in immune cell development, including B cells, after 1 wk of recovery on Earth. FASEB J. 33, 3772–3783 (2019).
Guéguinou, N. et al. Could spaceflight-associated immune system weakening preclude the expansion of human presence beyond Earth’s orbit? J. Leukoc. Biol. 86, 1027–1038 (2009).
Crucian, B., Stowe, R., Quiriarte, H., Pierson, D. & Sams, C. Monocyte phenotype and cytokine production profiles are dysregulated by short-duration spaceflight. Aviat. Space Environ. Med. 82, 857–862 (2011).
Tsai, S. et al. Insulin receptor-mediated stimulation boosts T cell immunity during inflammation and infection. Cell Metab. 28, 922–934.e4 (2018).
Jacob, P. et al. Next generation of astronauts or ESA astronaut 2.0 concept and spotlight on immunity. npj Microgravity 9, 51 (2023).
Chang, T. T. et al. The Rel/NF-κB pathway and transcription of immediate early genes in T cell activation are inhibited by microgravity. J. Leukoc. Biol. 92, 1133–1145 (2012).
Buchheim, J.-I. et al. Plasticity of the human IgM repertoire in response to long-term spaceflight. FASEB J. 34, 16144–16162 (2020).
Stratis, D., Trudel, G., Rocheleau, L., Pelchat, M. & Laneuville, O. The transcriptome response of astronaut leukocytes to long missions aboard the International Space Station reveals immune modulation. Front. Immunol. 14, 1171103 (2023). A comprehensive bulk transcriptomic analysis of PBMCs from 14 astronauts over a period of 6 months identifies DEGs altered by spaceflight, including pathways linked to lymphocyte activation, immune cell proliferation, cell adhesion, migration, wound response and GPCR signalling, as well as changes in long-non-coding RNAs.
Mucka, P. et al. CLK2 and CLK4 are regulators of DNA damage-induced NF-κB targeted by novel small molecule inhibitors. Cell Chem. Biol. 30, 1303–1312.e3 (2023).
Moreno-Villanueva, M. et al. Transcriptomics analysis reveals potential mechanisms underlying mitochondrial dysfunction and T cell exhaustion in astronauts’ blood cells in space. Front. Immunol. 15, 1512578 (2024). A large-scale bulk transcriptomic analysis of PBMCs from 11 astronauts at 135–201 days on board the ISS identifies key pathways and mechanisms imparted by spaceflight to alter immunity. Some of these include changes to mitochondria, sirtuin signalling, cytokines, mechanobiology and calcium-related processes and autophagy.
Overbey, E. G. et al. The Space Omics and Medical Atlas (SOMA) and international astronaut biobank. Nature 632, 1145–1154 (2024). SOMA is a pivotal database and biobanking resource providing comprehensive physiological profiles and multiomics datasets from a range of space missions to accelerate biomedical understanding and the development of critical countermeasures for the challenges of long-duration space travel.
Liu, H., He, R., Yang, X., Huang, B. & Liu, H. Mechanism of TCF21 downregulation leading to immunosuppression of tumor-associated macrophages in non-small cell lung cancer. Pharmaceutics 15, 2295 (2023).
Park, J. et al. Spatial multi-omics of human skin reveals KRAS and inflammatory responses to spaceflight. Nat. Commun. 15, 4773 (2024).
Kieffer, S. R. & Lowndes, N. F. Immediate-early, early, and late responses to DNA double stranded breaks. Front. Genet. 13, 793884 (2022).
Murray, D., Mirzayans, R. & McBride, W. H. Defenses against pro-oxidant forces — maintenance of cellular and genomic integrity and longevity. Radiat. Res. 190, 331–349 (2018).
Zhao, T. et al. Simulated microgravity promotes cell apoptosis through suppressing Uev1A/TICAM/TRAF/NF-κB-regulated anti-apoptosis and p53/PCNA- and ATM/ATR-Chk1/2-controlled DNA-damage response pathways. J. Cell. Biochem. 117, 2138–2148 (2016).
Magalhaes, Y. T., Boell, V. K., Cardella, G. D. & Forti, F. L. Downregulation of the Rho GTPase pathway abrogates resistance to ionizing radiation in wild-type p53 glioblastoma by suppressing DNA repair mechanisms. Cell Death Dis. 14, 283 (2023).
Shokrollahi, M. et al. DNA double-strand break-capturing nuclear envelope tubules drive DNA repair. Nat. Struct. Mol. Biol. 31, 1319–1330 (2024).
Luxton, J. J. et al. Telomere length dynamics and DNA damage responses associated with long-duration spaceflight. Cell Rep. 33, 108457 (2020).
George, K., Rhone, J., Beitman, A. & Cucinotta, F. A. Cytogenetic damage in the blood lymphocytes of astronauts: effects of repeat long-duration space missions. Mutat. Res. 756, 165–169 (2013).
Schmidt, S. et al. Glucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death Differ. 11, S45–S55 (2004).
ElGindi, M. et al. May the force be with you (or not): the immune system under microgravity. Cells 10, 1941 (2021).
Casalino-Matsuda, S. M. et al. Myeloid Zfhx3 deficiency protects against hypercapnia-induced suppression of host defense against influenza A virus. JCI Insight 9, e170316 (2024).
Mehta, S. K. et al. Antiviral treatment with valacyclovir reduces virus shedding in saliva of Antarctic expeditioners. Front. Virol. 3, 1157659 (2023).
Makedonas, G. et al. Specific immunologic countermeasure protocol for deep-space exploration missions. Front. Immunol. 10, 2407 (2019).
Rettig, T. A., Tan, J. C., Nishiyama, N. C., Chapes, S. K. & Pecaut, M. J. An analysis of the effects of spaceflight and vaccination on antibody repertoire diversity. Immunohorizons 5, 675–686 (2021).
Jones, C. W. et al. Molecular and physiological changes in the SpaceX Inspiration4 civilian crew. Nature 632, 1155–1164 (2024).
An, R. et al. Influence of the spaceflight environment on macrophage lineages. npj Microgravity 10, 63 (2024).
Overbey, E. G. et al. Collection of biospecimens from the Inspiration4 mission establishes the standards for the Space Omics and Medical Atlas (SOMA). Nat. Commun. 15, 4964 (2024).
Garcia-Medina, J. S. et al. Genome and clonal hematopoiesis stability contrasts with immune, cfDNA, mitochondrial, and telomere length changes during short duration spaceflight. Precis. Clin. Med. 7, pbae007 (2024).
Jones, H. W. The partial gravity of the Moon and Mars appears insufficient to maintain human health. In 50th International Conference on Environmental Systems (ICES, 2021).
Richter, C., Braunstein, B., Winnard, A., Nasser, M. & Weber, T. Human biomechanical and cardiopulmonary responses to partial gravity — a systematic review. Front. Physiol. 8, 583 (2017).
Taylor, C. T. & Scholz, C. C. The effect of HIF on metabolism and immunity. Nat. Rev. Nephrol. 18, 573–587 (2022).
Benavides Damm, T., Walther, I., Wüest, S. L., Sekler, J. & Egli, M. Cell cultivation under different gravitational loads using a novel random positioning incubator. Biotechnol. Bioeng. 111, 1180–1190 (2014).
Miranda, S. et al. A dusty road for astronauts. Biomedicines 11, 1921 (2023).
Sun, Y. et al. Research on rat’s pulmonary acute injury induced by lunar soil simulant. J. Chin. Med. Assoc. 81, 133–140 (2018).
Sun, Y. et al. Mechanisms involved in inflammatory pulmonary fibrosis induced by lunar dust simulant in rats. Environ. Toxicol. 34, 131–140 (2019).
Lam, C.-W. et al. Comparative pulmonary toxicities of lunar dusts and terrestrial dusts (TiO2 & SiO2) in rats and an assessment of the impact of particle-generated oxidants on the dusts’ toxicities. Inhal. Toxicol. 34, 51–67 (2022).
Horie, M., Miki, T., Honma, Y., Aoki, S. & Morimoto, Y. Evaluation of cellular effects caused by lunar regolith simulant including fine particles. J. UOEH 37, 139–148 (2015).
Li, M. et al. Lunar soil simulants alter macrophage survival and function. J. Appl. Toxicol. 39, 1413–1423 (2019).
Zhou, H. et al. NLRP3 Inflammasome mediates silica-induced lung epithelial injury and aberrant regeneration in lung stem/progenitor cell-derived organotypic models. Int. J. Biol. Sci. 19, 1875–1893 (2023).
Wang, J. et al. Physical activation of innate immunity by spiky particles. Nat. Nanotechnol. 13, 1078–1086 (2018).
Caston, R., Luc, K., Hendrix, D., Hurowitz, J. A. & Demple, B. Assessing toxicity and nuclear and mitochondrial DNA damage caused by exposure of mammalian cells to lunar regolith simulants. GeoHealth 2, 139–148 (2018).
Iosim, S., MacKay, M., Westover, C. & Mason, C. E. Translating current biomedical therapies for long duration, deep space missions. Precis. Clin. Med. 2, 259–269 (2019).
Nangle, S. N. et al. The case for biotech on Mars. Nat. Biotechnol. 38, 401–407 (2020).
Evans, M. E. & Graham, L. D. A Flexible Lunar Architecture for Exploration (FLARE) supporting NASA’s Artemis program. Acta Astronaut. 177, 351–372 (2020).
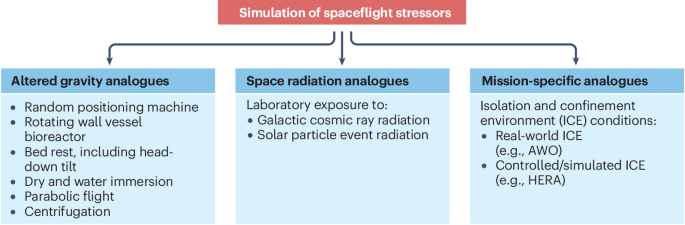
Hoson, T., Kamisaka, S., Masuda, Y., Yamashita, M. & Buchen, B. Evaluation of the three-dimensional clinostat as a simulator of weightlessness. Planta 203, S187–S197 (1997).
Kiss, J. Z., Wolverton, C., Wyatt, S. E., Hasenstein, K. H. & van Loon, J. J. W. A. Comparison of microgravity analogs to spaceflight in studies of plant growth and development. Front. Plant. Sci. 10, 1577 (2019).
Vashi, A., Sreejith, K. R. & Nguyen, N.-T. Lab-on-a-chip technologies for microgravity simulation and space applications. Micromachines 14, 116 (2022).
Anil-Inevi, M. et al. Biofabrication of in situ self assembled 3D cell cultures in a weightlessness environment generated using magnetic levitation. Sci. Rep. 8, 7239 (2018).
Marycz, K., Kornicka, K. & Röcken, M. Static magnetic field (SMF) as a regulator of stem cell fate — new perspectives in regenerative medicine arising from an underestimated tool. Stem Cell Rev. Rep. 14, 785–792 (2018).
Morey-Holton, E. R. & Globus, R. K. Hindlimb unloading rodent model: technical aspects. J. Appl. Physiol. 92, 1367–1377 (2002).
Morey-Holton, E., Globus, R. K., Kaplansky, A. & Durnova, G. Experimentation with Animal Models in Space 107–140 (Elsevier, 2005).
Mortreux, M., Nagy, J. A., Ko, F. C., Bouxsein, M. L. & Rutkove, S. B. A novel partial gravity ground-based analog for rats via quadrupedal unloading. J. Appl. Physiol. 125, 175–182 (2018).
Pavy-Le Traon, A., Heer, M., Narici, M. V., Rittweger, J. & Vernikos, J. From space to Earth: advances in human physiology from 20 years of bed rest studies (1986–2006). Eur. J. Appl. Physiol. 101, 143–194 (2007).
Saveko, A. et al. Impact of different ground-based microgravity models on human sensorimotor system. Front. Physiol. 14, 1085545 (2023).
Hemmersbach, R., Häder, D.-P. & Braun, M. Gravitational Biology I: Gravity Sensing and Graviorientation in Microorganisms and Plants 13–26 (Springer International Publishing, 2018).
Cucinotta, F. A., Kim, M.-H. Y., Chappell, L. J. & Huff, J. L. How safe is safe enough? Radiation risk for a human mission to Mars. PLoS ONE 8, e74988 (2013).
Afshinnekoo, E. et al. Fundamental biological features of spaceflight: advancing the field to enable deep-space exploration. Cell 183, 1162–1184 (2020).
Borovsky, J. E., Halekas, J. S. & Whittlesey, P. L. The electron structure of the solar wind. Front. Astron. Space Sci. 8, 690005 (2021).
Durante, M. & Cucinotta, F. A. Physical basis of radiation protection in space travel. Rev. Mod. Phys. 83, 1245–1281 (2011).
Dobney, W. et al. Evaluation of deep space exploration risks and mitigations against radiation and microgravity. Front. Nucl. Med. 3, 1225034 (2023).
Huff, J. L. et al. Galactic cosmic ray simulation at the NASA Space Radiation Laboratory — progress, challenges and recommendations on mixed-field effects. Life Sci. Space Res. 36, 90–104 (2023).
Norbury, J. W. et al. Galactic cosmic ray simulation at the NASA Space Radiation Laboratory. Life Sci. Space Res. 8, 38–51 (2016).
Feuerecker, M. et al. Immune sensitization during 1 year in the Antarctic high-altitude Concordia environment. Allergy 74, 64–77 (2019).
Buchheim, J.-I. et al. Exploratory RNA-seq analysis in healthy subjects reveals vulnerability to viral infections during a 12-month period of isolation and confinement. Brain Behav. Immun. Health 9, 100145 (2020).
Ngo-Anh, T. J., Rossiter, A., Suvorov, A., Vassilieva, G. & Gushin, V. in Stress Challenges and Immunity in Space: From Mechanisms to Monitoring and Preventive Strategies (ed. Choukér, A.) 677–692 (Springer International Publishing, 2020).
Campisi, M., Cannella, L. & Pavanello, S. Cosmic chronometers: is spaceflight a catalyst for biological ageing? Ageing Res. Rev. 95, 102227 (2024).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Baechle, J. J. et al. Chronic inflammation and the hallmarks of aging. Mol. Metab. 74, 101755 (2023).
Al-Turki, T. M. et al. Telomeric RNA (TERRA) increases in response to spaceflight and high-altitude climbing. Commun. Biol. 7, 698 (2024).
Ross, J. B. et al. Depleting myeloid-biased haematopoietic stem cells rejuvenates aged immunity. Nature 628, 162–170 (2024).
Pioli, P. D., Casero, D., Montecino-Rodriguez, E., Morrison, S. L. & Dorshkind, K. Plasma cells are obligate effectors of enhanced myelopoiesis in aging bone marrow. Immunity 51, 351–366.e6 (2019).
Bisserier, M. et al. Cell-free mitochondrial DNA as a potential biomarker for astronauts’ health. J. Am. Heart Assoc. 10, e022055 (2021).
Nidadavolu, L. S. et al. Associations between circulating cell-free mitochondrial DNA, inflammatory markers, and cognitive and physical outcomes in community dwelling older adults. Immun. Ageing 20, 24 (2023).
Buchheim, J.-I. et al. Stress related shift toward inflammaging in cosmonauts after long-duration space flight. Front. Physiol. 10, 85 (2019). This study, which monitored 12 cosmonauts during long-duration spaceflight, found a sustained stress response characterized by increased endocannabinoids and aberrant immune activation, with signs of inflammation with some similarities to ageing, evident in altered lymphocyte percentages, cytokine levels and T cell repertoire.
ElGindi, M. et al. Effects of an aged tissue niche on the immune potency of dendritic cells using simulated microgravity. npj Aging 9, 14 (2023).
Johnston, S. L. Flight crew health stabilization program. Space Medicine Division (2010).
Petersen, E. et al. Adapting disease prevention protocols for human spaceflight during COVID-19. Aerosp. Med. Hum. Perform. 92, 597–602 (2021).
Makedonas, G., Mehta, S. K., Scheuring, R. A., Haddon, R. & Crucian, B. E. SARS-CoV-2 pandemic impacts on NASA ground operations to protect ISS astronauts. J. Allergy Clin. Immunol. Pract. 8, 3247–3250 (2020).
Crucian, B. et al. A case of persistent skin rash and rhinitis with immune system dysregulation onboard the International Space Station. J. Allergy Clin. Immunol. Pract. 4, 759–762.e8 (2016).
Mehta, S. K. et al. Immune system dysregulation preceding a case of laboratory-confirmed zoster/dermatitis on board the International Space Station. J. Allergy Clin. Immunol. Glob. 3, 100244 (2024).
Krieger, S. S. et al. Alterations in saliva and plasma cytokine concentrations during long-duration spaceflight. Front. Immunol. 12, 725748 (2021).
Agha, N. H. et al. Exercise as a countermeasure for latent viral reactivation during long duration space flight. FASEB J. 34, 2869–2881 (2020).
Diaz, T. E., Ives, E. C., Lazare, D. I. & Buckland, D. M. Expiration analysis of the International Space Station formulary for exploration mission planning. npj Microgravity 10, 76 (2024).
Crucian, B. et al. Spaceflight validation of technology for point-of-care monitoring of peripheral blood WBC and differential in astronauts during space missions. Life Sci. Space Res. 31, 29–33 (2021).
Space Station Research Investigation. NASA mission database entry [Online] https://www.nasa.gov/mission/station/research-explorer/investigation/?#id=8821 (accessed 2025).
Dubeau-Laramée, G., Rivière, C., Jean, I., Mermut, O. & Cohen, L. Y. Microflow1, a sheathless fiber-optic flow cytometry biomedical platform: demonstration onboard the international space station. Cytom. A 85, 322–331 (2014).
Crucian, B. & Sams, C. Reduced gravity evaluation of potential spaceflight-compatible flow cytometer technology. Cytom. B Clin. Cytom. 66, 1–9 (2005).
Rea, D. J. et al. Single drop cytometry onboard the International Space Station. Nat. Commun. 15, 2634 (2024).



![Secondary infections caused by bacteria or viruses arising during in-hospital care remain a long-sta.. [Pixabay]](https://www.vitaminrush.com/wp-content/uploads/2026/02/1770772115_news-p.v1.20260211.ead5f668530548ee9372784a8cab52f4_P1.jpg)