image:
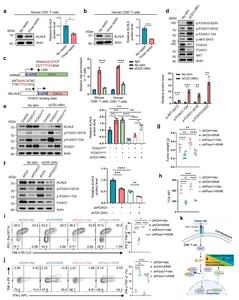
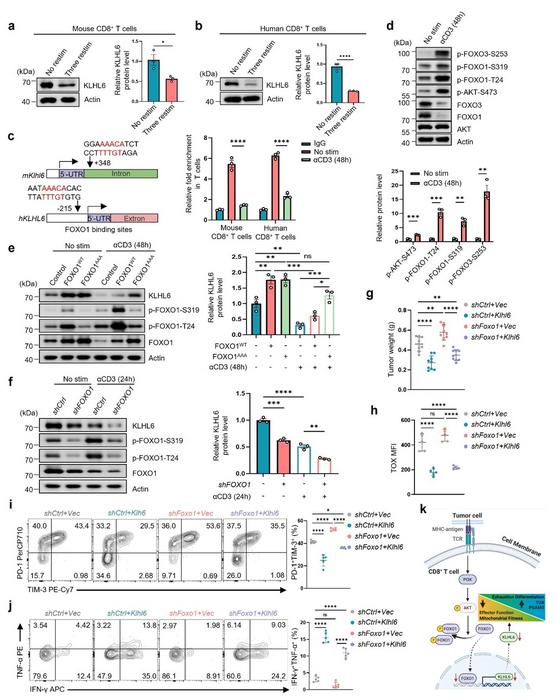
a-b Immunoblots and quantification of KLHL6 expression in mouse and human CD8⁺ T cells after TCR stimulation. Splenic OT-I CD8⁺ T cells were initially activated by anti-CD3/CD28 antibodies, expanded in the media containing mIL-2 for 4 days, and then either three repeated stimulations in vitro using anti-CD3 (2 μg/mL) antibody treatment every 2 days until day 10 (Three restim), or no restimulations and let the cell rest in mIL-2 media until day 10 (No restim) (a). For human T cells, the CD8⁺ T cells were activated by anti-CD3/CD28 antibodies and expanded in the media containing hIL-2 for 8 days, and then either three repeated stimulations (Three restim) in vitro using anti-CD3 (2 μg/mL) antibody treatment every 2 days until day 14, or no restimulations and let the cell rest in hIL-2 media until day 14 (No restim) (b). n = 3 independent samples. c Binding sites of FOXO1 to the promoter of Klhl6 analyzed using JASPAR (left). FOXO1 occupancy on the conserved region of Klhl6 promoter in both mouse and human CD8+ T cells was analyzed (right). The activated mouse and human T cells were cultured for 4 days and then re-stimulated with anti-CD3 (2 μg/mL) antibody for 2 days. The cells with (anti-CD3) or without (No stim) re-stimulation were collected and analyzed using CUT&RUN-qPCR. IgG: negative control. n = 3 independent samples. d Assessment of AKT-FOXO axis by western blot in resting or stimulated OT-I T cells. The activated OT-I CD8+ T cells were cultured for 4 days in vitro and then re-stimulated by anti-CD3 (2 μg/mL) antibody for 2 days. The cells re-stimulated (with anti-CD3) or not re-stimulated (No stim) were collected and analyzed. n = 3 independent samples. e Immunoblot and quantification of KLHL6 in OT-I CD8⁺ T cells transduced with FOXO1WT, FOXO1AAA, or empty plasmid (Ctrl). The transduced cells were cultured for 4 days in vitro, and then re-stimulated with anti-CD3 (2 μg/mL) for 48 h for analysis. n = 3 independent samples. f Immunoblot and quantification of KLHL6 expression in shFOXO1 or shCtrl Jurkat cells after stimulation with anti-CD3 (2 μg/mL) for 24 h. n = 3 independent samples. g-j CD45.1+ OT-I CD8⁺ T cells were transduced with indicated plasmids, and adoptively transferred into CD45.2⁺ mice bearing B16-OVA tumor that had been implanted 9 days earlier. Tumor weights in tumor-bearing mice were measured on day 14 after ACT (g, n = 9 mice); TOX expression in transferred CD8⁺ TILs at day 14 after ACT (h, n = 5 mice). Representative plots (left) and percentages (right) of TIM-3⁺PD-1⁺ populations in transferred CD8⁺ TILs at day 14 after ACT (i, n = 5 mice); TNF-α and IFN-γ production in transferred CD8⁺ TILs after 4.5 h PMA + BFA stimulation at day 14 after ACT (j, n = 5 mice). K Schematic diagram illustrates chronic TCR engagement promoting CD8⁺ T cell exhaustion. Data are presented as mean ± SEM. Statistical analyses were determined by unpaired two-tailed Student’s t-test (a-d) or two-way ANOVA with Tukey’s multiple-comparisons test (e-j). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001; ns, no significance
Credit: Prof. Guideng Li from Suzhou Institute of Systems Medicine, China
Image source link: https://link.springer.com/article/10.1007/s44466-025-00023-z
CD8⁺ T cells are the frontline soldiers of the body against cancer, but their prolonged exposure to tumor antigens often leads to an exhausted state. This state is marked by weakened function, increased expression of inhibitory receptors, and profound epigenetic and metabolic reprogramming. While a subset known as precursor exhausted T cells retains some stem-like qualities and can respond to immunotherapy, terminally exhausted T cells are largely refractory to treatment. Preventing or reversing the progression to terminal exhaustion remains a central challenge.
To provide a mechanistic answer, a team of researchers led by Dr. Li Guideng from the Suzhou Institute of Systems Medicine, China, in collaboration with Dr. Philip D. Greenberg’s team at the Fred Hutchinson Cancer Center, USA, published two complementary papers. The first, titled “Chronic TCR Signaling-Driven Suppression of the FOXO1-KLHL6 Axis Promotes T Cell Exhaustion,” was made available online on January 14, 2026, in the journal Immunity & Inflammation. The other, titled “The Ubiquitin Ligase KLHL6 Drives Resistance to CD8+ T Cell Dysfunction,” was published in the journal Nature on January 14, 2026. They demonstrate that persistent T cell receptor (TCR) signaling, instead of sustaining activation, acts as a master switch by suppressing the transcription factor FOXO1. This suppression leads to the sustained downregulation of an E3 ubiquitin ligase, KLHL6, a previously unrecognized pivotal event in initiating the exhaustion program.
Through integrative analysis of T cell exhaustion data from viral infection and tumor models, the researchers first identified dysregulation in protein degradation pathways as reported in the study in Nature. A subsequent computational-guided CRISPR screen pinpointed KLHL6 as a key factor capable of simultaneously suppressing exhaustion and improving mitochondrial function. Mechanistically, KLHL6 was found to ubiquitinate and target two key proteins for degradation: TOX, a master transcription factor of exhaustion, and PGAM5, a regulator of mitochondrial dynamics. Under chronic stimulation, decreased KLHL6 levels allow TOX to accumulate—accelerating exhaustion—and cause PGAM5 buildup, which promotes excessive mitochondrial fragmentation (via Drp1) and metabolic dysfunction.
The companion study in Immunity & Inflammation solved the upstream puzzle: How does chronic TCR signaling turn off KLHL6? The team discovered that acute TCR signaling transiently suppresses KLHL6 via the PI3K-AKT pathway, which phosphorylates and inhibits FOXO1, a transcription factor that binds to the KLHL6 promoter. Critically, under persistent antigen stimulation, FOXO1 activity is chronically suppressed, leading to irreversible downregulation of KLHL6 and locking T cells into the exhaustion pathway.
The research further established the functional hierarchy within this axis. While FOXO1 is known to promote memory formation in CAR-T cells, the team found that KLHL6 overexpression could rescue the anti-tumor function and memory potential of FOXO1-deficient T cells. “This indicates that KLHL6 is a major downstream executor of FOXO1’s beneficial effects on T cell fitness,” the authors noted.
These findings establish the FOXO1-KLHL6 axis as a core regulator translating chronic antigen exposure into a state of T cell exhaustion. They offer a mechanistic explanation for a long-standing paradox in immunology and reveal KLHL6 as a highly promising therapeutic target. “Strategies to boost KLHL6 activity or mimic its function—such as developing protein degraders targeting TOX/PGAM5 or KLHL6 agonists—could potentially prevent or reverse T cell exhaustion, thereby enhancing the efficacy of existing immunotherapies like immune checkpoint blockade, CAR-T, and TCR-T cell therapies,” the authors highlighted.
***
Reference
DOI: 10.1007/s44466-025-00023-z
DOI: 10.1038/s41586-025-09926-8
About Immunity & Inflammation
Immunity & Inflammation is a newly launched open-access journal co-published by the Chinese Society for Immunology and Springer Nature under the leadership of Editors-in-Chief Prof. Xuetao Cao and Prof. Jules A. Hoffmann. Immunity & Inflammation aims to publish major scientific questions and cutting-edge advances that explore groundbreaking discoveries and insights across the spectrum of immunity and inflammation, from basic science to translational and clinical research.
Website: https://link.springer.com/journal/44466
About authors
Professor Guideng Li from Suzhou Institute of Systems Medicine, China
Professor Guideng Li is a Principal Investigator and Deputy Director at the Suzhou Institute of Systems Medicine. He leads multiple national research projects, including grants from the National Natural Science Foundation of China. His research focuses on the molecular mechanisms of tumor antigen-specific T cell immunity and the development of novel intervention strategies.
Dr. Hongcheng Cheng from Suzhou Institute of Systems Medicine, China
Dr. Hongcheng Cheng is an Associate Investigator at the Suzhou Institute of Systems Medicine. His research focuses on the regulatory mechanisms of T cell exhaustion and strategic interventions.
Funding information
This work was supported by the Noncommunicable Chronic Diseases-National Science and Technology Major Project (2024ZD0520600), the National Natural Science Foundation of China (32525028 and 32270994 to Guideng Li, 32571074 and 32300764 to Hongcheng Cheng), the Basic Research Program of Jiangsu (BK20250003 to Guideng Li, BK20230280 to Hongcheng Cheng), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2021-RC310-014 and 2024-JKCS-15 to Guideng Li), the CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-047, 2021-I2M-1-061, 2022-I2M-2-004, 2023-I2M-2-010, 2023-I2MQJ-019, 2024-I2M-ZD-009, and 2025-I2M-GCC-006 to Guideng Li, 2025-I2M-TS-15 to Hongcheng Cheng).
Method of Research
Experimental study
Subject of Research
Animals
Article Title
Chronic TCR signaling-driven suppression of the FOXO1-KLHL6 axis promotes T cell exhaustion
Article Publication Date
14-Jan-2026
COI Statement
The corresponding author Guideng Li is a member of the Editorial Board of the journal Immunity & Inflammation. However, he was not involved in the peer-review or decision-making process for this manuscript. The authors declare no other competing interests.
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.