Study design and participants
This study employed a randomized, double-blind, placebo-controlled design. Participants were recruited from male students majoring in physical education at Beijing Sport University, China. The recruitment criteria included individuals aged 18 to 28 years. The International Physical Activity Questionnaire (IPAQ) was used to assess participants’ habitual physical activity levels.
Sample size estimation was conducted using G*Power software (version 3.1.9.7; Heinrich Heine University, Düsseldorf, Germany). An a priori effect size (f) of 0.25 was specified, with a significance level of α = 0.05 and a statistical power of 0.80. The design included four groups and two repeated measurements. Based on these inputs, G*Power estimated a required total sample size of 48 participants (12 per group). To account for potential dropouts, an additional 3 participants were recruited for each group.
The inclusion criteria were as follows: (a) a Body Mass Index (BMI) between 18.5 and 24 kg/m2; (b) regular exercise habits over the preceding three months, defined as engaging in physical exercise at least three times per week, with each session lasting at least 30 min and of moderate or higher intensity; (c) non-consumption of coffee, tobacco products, and alcohol; (d) absence of cardiopulmonary disease; (e) absence of metabolic diseases such as endocrine, kidney, or gastrointestinal disorders; (f) absence of sports-related injuries and movement disorders; (g) no medication use in the month prior to enrollment; and (h) no participation in any other clinical nutrition research trials.
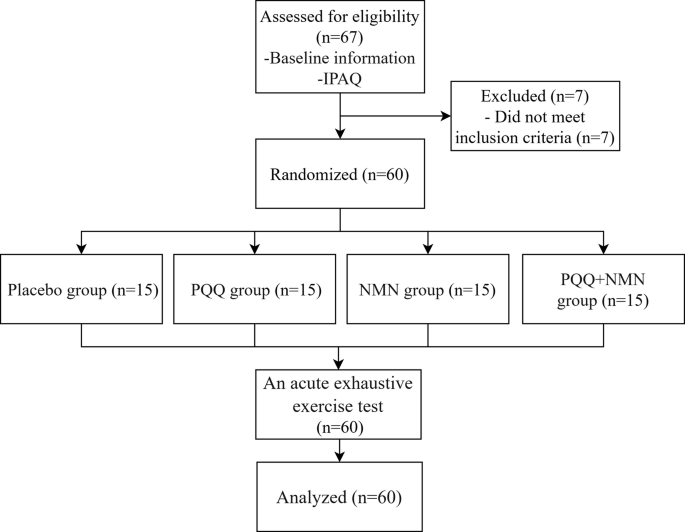
All participants provided written informed consent after receiving a detailed explanation of the study’s purpose and procedures. The study protocol was approved by the Scientific Ethics Committee at Beijing Sport University (approval number: 2023286H, approval date: 14 November 2023). This study was retrospectively registered in the Chinese Clinical Trial Registry (ChiCTR2500112623) on 17 November 2025. Baseline data, including age, sex, athletic classification, smoking status, alcohol consumption habits, and general health status, were collected via a baseline survey and physical examination. Sixty participants met the eligibility criteria and were subsequently enrolled in the study. Participants were randomly assigned to one of four treatment groups using a computer-generated random allocation sequence: the PQQ supplement group (PQQ, n = 15), the NMN supplement group (NMN, n = 15), the PQQ with NMN supplement group (PN, n = 15), or the placebo group (PLC, n = 15). Figure 1 shows the process of participant selection and group allocation.
The process of participant selection and group allocation.
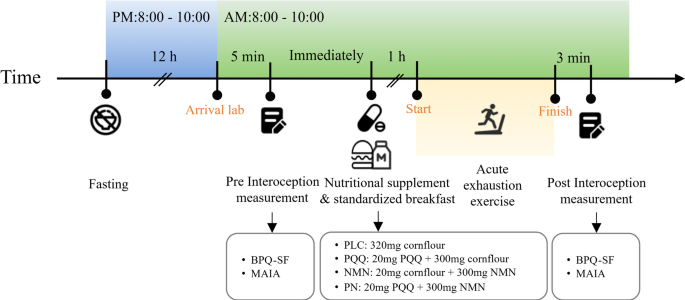
To ensure compliance and dietary consistency, participants were instructed to abstain from vitamins, minerals, coffee, tea, and other supplements throughout the study period. All participants completed a 48-h dietary recall questionnaire and were required to complete for 12-h overnight fast prior to the experimental trial. Participants arrived at the laboratory in the morning. After a 5-min rest period, they completed the baseline interoception questionnaire assessment. Subsequently, they consumed the assigned nutritional supplement along with a standardized breakfast (a 400 kcal hamburger and 250 mL of milk). After one hour, participants performed an acute exhaustive exercise test. Upon completion of the exercise, participants walked on a treadmill at 3 km/h for 3 min, followed by a second interoception questionnaire assessment. Figure 2 presents an illustration of the experimental protocol for testing sessions in the lab.
The experimental protocol.
Acute exhaustion exercise protocol
All participants in the four intervention groups completed an acute exhaustion exercise test following the Bruce incremental loading protocol on a treadmill (RUN 7410, Runner, Italy). Expired gases were continuously monitored using a gas analyser (Metalyzer 3B, CORTEX Biophysik, Germany). Heart rate was continuously recorded using a Firstbeat Sports Heart Rate Band (Firstbeat Sports, Firstbeat Technologies, Finland). The protocol consisted of running on the treadmill until volitional exhaustion.
The treadmill started at an initial speed of 2.74 km/h with a 10% slope. Speed and slope were increased incrementally every 3 min29. At the point of exhaustion, participants verbally reported their perceived level of fatigue using the Borg 6–20 Rating of Perceived Exertion (RPE) scale.
The experiment was terminated immediately when participants reached volitional exhaustion. The criteria for determining exhaustion were as follows: (1) participant reported feeling exhausted and was unable to continue the exercise despite verbal encouragement; (2) oxygen uptake had plateaued (no longer changed or decreased) with further increases in the exercise workload; (3) the participant’s heart rate (HR) was at or near their predicted maximal heart rate (HRmax); and (4) the respiratory exchange ratio (RER) was ≥ 1.10.
Supplementation protocol and blinding
In a double-blind fashion, participants were randomly assigned to orally ingest 320 mg of cornflour (PLC), 20 mg of PQQ with 300 mg of cornflour (PQQ), 300 mg of NMN with 20 mg of cornflour (NMN), or 20 mg of PQQ with 300 mg of NMN (PN). The PQQ used was provided in the form of PQQ disodium. All supplements were manufactured to be identical in size, shape, and colour. One researcher, who alone was aware of the allocation, distributed the supplements in a randomised manner and kept the records. This researcher was not involved in testing or data analysis, thereby ensuring blinding of both participants and investigators. Study personnel distributed the respective supplement to each participant 60 min before the exercise session.
Interoception measurement
Interoceptive sensitivity was measured using subjective self-report questionnaires. The Body Perception Questionnaire (BPQ) and the Multidimensional Assessment of Interoceptive Awareness (MAIA) are two commonly cited questionnaires in interoception research30. Moreover, both questionnaires have been validated for reliability and validity in Chinese university student populations31,32. Therefore, interoception was measured using the BPQ and MAIA questionnaires.
The body perception questionnaire-short form (BPQ-SF)
The BPQ-SF is a shortened version of the BPQ’s Body Awareness subscale33. The BPQ-SF consists of 46 items measuring sensitivity to internal bodily sensations and autonomic nervous responses. Item responses are scored on a 5-point ordinal scale ranging from 1 (never) to 5 (always). The overall BPQ-SF score was calculated by summing all item scores.
The multidimensional assessment of interoceptive awareness (MAIA) 2nd edition
The MAIA consists of 37 items divided into eight subscales: Noticing, Not-Worrying, Not-Distracting, Attention Regulation, Emotional Awareness, Self-Regulation, Body Listening, and Trusting34. Each item is scored on a 6-point Likert scale ranging from 0 (never) to 5 (always), with higher scores indicating greater self-reported interoceptive awareness. The Not-Worrying and Not-Distracting subscales are reverse-scored. Scores for each MAIA subscale were determined by averaging the scores of all items within the respective subscale.
Exercise capacity measurement
Gas analysers (Metalyzer 3B, CORTEX Biophysik, Germany) were used to measure exercise capacity during the acute exhaustive exercise test. Baseline values were obtained from an exhaustive exercise test using the same treadmill protocol as in the formal experiment, administered during the participant recruitment phase. Participants underwent the Bruce protocol to determine their time to exhaustion (TTE), time to the anaerobic threshold (AT), peak respiratory exchange ratio (RER), maximal oxygen uptake (VO2max), VO2 at AT, VO2 utilisation (VO2 at AT/VO2max), and respiratory efficiency (VEmax/VO2max). These variables were used as indicators of exercise capacity.
Statistical analysis
Data were analysed using the Statistical Package for the Social Sciences software (SPSS Version 25.0, IBM Corporation, Armonk, NY, USA). Normality and homogeneity of variance were tested (p > 0.05). A chi-square test and one-way analysis of variance (ANOVA) were used to examine differences in participants’ characteristics (age, BMI, and athletic classification) across the study groups. A mixed-design ANOVA was then applied to assess differences in interoception between the four groups, both before and after exercise. Analysis of covariance (ANCOVA) was used to examine the differences in exercise capacity indicators among the four groups after adjusting for baseline exercise capacity, age, BMI, and athlete classification. Indicators with significant differences among the groups were further analyzed using Bonferroni post-hoc multiple comparisons to assess pairwise differences between the two groups. Partial correlation analysis was conducted to explore the correlation between exercise performance (RPE, VO2max, and HRmax) and changes in interoception (post values minus pre values), while adjusting for the groups, age, BMI, and athlete classification. In addition, Partial eta square (ηp2) effect sizes (ES) were calculated to compare the magnitude of differences in interoception among the groups. ηp2 thresholds of 0.01, 0.06, and 0.14 were interpreted as small, moderate, and large effects, respectively. The level of significance was set at α = 0.05.