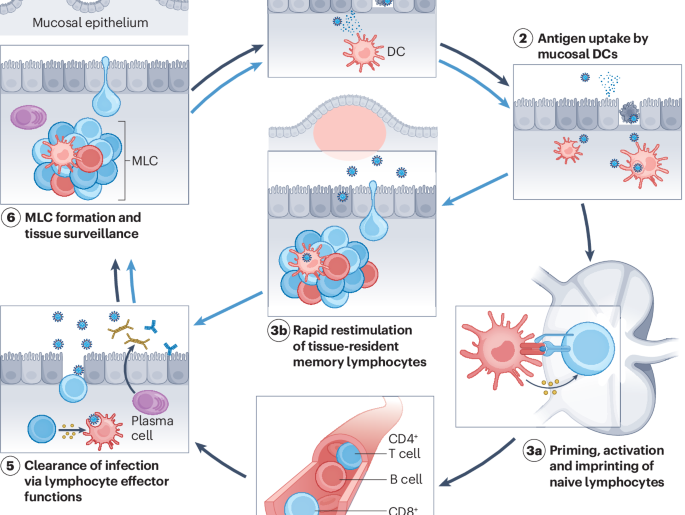
In a significant step forward for mucosal immunity, Aptar Pharma has announced that its innovative nasal delivery systems are being utilized in CastleVax’s Phase II clinical trial for CVAX-01, an intranasal COVID-19 vaccine candidate.
The trial is designed to evaluate the safety and immunogenicity of the vaccine, which aims to provide a more robust immune response at the site of infection (the respiratory tract) than traditional systemic injections. To ensure precise and effective administration, the study utilizes Aptar’s LuerVax™, a specialized syringe-to-nasal spray adapter, and their Spray Divider™ technology, which allows for accurate bilateral dosing.
This collaboration highlights a growing industry shift toward needle-free delivery. By leveraging Aptar’s “Pharma Services” platform, CastleVax is able to combine its vaccine candidate with proven device technology that meets rigorous regulatory and technical standards. This holistic approach is essential for navigating the complexities of nasal formulation, from device compatibility to patient-centric design.
We are pleased to support CastleVax in this pivotal trial, Our goal is to help our partners accelerate the delivery of life-changing therapies by providing reliable, high-performance nasal solutions.”
Alex Theodorakis, President, Aptar Pharma Prescription
The success of such trials continues to drive momentum in the respiratory and systemic drug delivery space. For professionals interested in the technical evolution of these delivery platforms and the latest advancements in mucosal vaccination, these topics will be at the forefront of the upcoming Nasal Formulation and Delivery Summit.
As a key sponsor of the event, Aptar Pharma will be joining other industry leaders to discuss the future of the field. The event offers a unique opportunity to explore the science behind these clinical breakthroughs and network with the experts who are shaping the next generation of intranasal medicine.