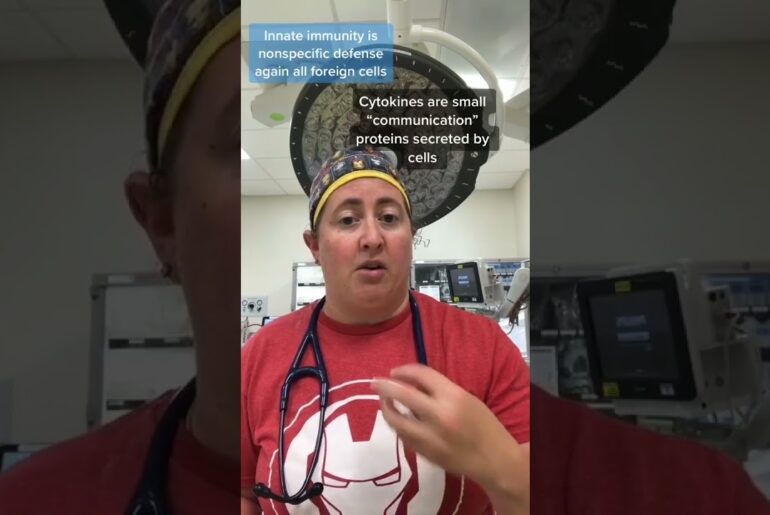
“Vielight RX Plus” PBM device is a possible treatment for COVID-19. We are hypothesizing that the RX Plus would help to shorten the disease duration, while improving the immune system’s response. – Dr. Lew Lim
The Vielight study will test a total of 280 subjects. These subjects will be randomized into a standard of care (SOC) group and a SOC plus treatment group. We will be monitoring the groups and measuring how well the RX Plus can help a person with COVID-19 to recover. We are hypothesizing that the RX Plus would help to shorten the disease duration, while improving the immune system’s response.”
Dr. Lim adds, “Once we obtain consent from the regulatory bodies to move forward, we will inform the public on how they can participate in the study. For logistical reasons, our clinical trial will be confined to the province of Ontario, Canada.”
Vielight RX Plus PBM device COVID-19 study
The Cure & Cause of Cancer
The Cure & Cause of Cancer
655 Prime Red available 10% Discount off with code serrano at
Disclaimer: These statements have not been evaluated by the FDA. The devices are low-risk general wellness products that do not require FDA clearance according to the FDA’s draft on “General Wellness: Policy on Low Risk Devices” – dated January 20, 2015.
The United States Patent and Trademark Office (USPTO) has issued a patent to Dr. Lew Lim, Founder & CEO of Vielight Inc, for the invention of the Vielight Neuro (that also covers all Vielight models). The patent is titled, “METHOD, SYSTEM AND APPARATUS FOR NON-INVASIVE NEUROSTIMULATION THERAPY OF THE BRAIN”. Its number is: US 10,632,325 B2, and the priority date of November 23, 2015. Vielight has full rights to use this technology. The worldwide patent, covering several other countries, is pending.